If a patient is confirmed to have HCV infection, the doctor must first assess his health status. Thanks to innovative laboratory diagnostic technologies, it is possible to analyze the likelihood of complications and determine how active the pathological process is. One of the important diagnostic parameters is the viral load for hepatitis C. Experts refer to this indicator as “viremia.”
Viral load indicators are also required to be known in order to develop antiviral therapy for the patient. Viremia should be determined before starting medication, then the test should be repeated two more times during the course of treatment. A steady downward trend indicates a good response to the selected medications. If the viral load remains at the same level or an increase is noted (compared to the first analysis), it is necessary to quickly change the treatment regimen or stop taking medications until the causes of resistance are identified.
Basic facts about hepatitis C
The provocateur of this disease is the hepatitis C virus, which infects liver cells and disrupts its functionality. The pathology can be acute or chronic.
In the acute stage, the patient has a chance of complete relief from the disease. However, this is possible if the diagnosis was timely and the treatment was adequate. Unfortunately, even in such cases it is not always possible to achieve positive results.
In 80% of patients, the immune system cannot fight the pathogenic agent: the disease progresses from the acute stage to the chronic stage and it is no longer possible to fully recover.
The hepatitis C pathogen cannot exist without the human body. Infection occurs only through blood: during a transfusion, when visiting a dentist, or through contact between open wounds of an infected person and a healthy person.
What is hepatitis C?
The hepatitis C virus was discovered relatively recently. If left untreated, the disease can progress to liver cirrhosis or cancer. Until a certain time, it was widely believed that this was a death sentence. This is all due to the low medical literacy of the population, as well as the fact that for a very long time medications for this disease were very expensive and inaccessible to the average person. However, now the cost of treatment is also quite high. However, it is possible to recover now.
The incubation period is up to 60 days. The main difficulty in diagnosing hepatitis C at the first stage is that symptoms may be mild or completely absent. In addition, symptoms may masquerade as other diseases. In general, the disease in its early stages can manifest itself as follows:
- Increased body temperature.
- Aches in the joints.
- Heaviness in the stomach area.
- Painful sensations in the right hypochondrium.
- Fast fatiguability.
- Digestive disorders.
- Stool or urine that is too light.
- General weakness.
The peculiarity of the disease is that all these signs appear separately and from time to time and pass relatively quickly. Therefore, detection in the early stages is very difficult. Therefore, it is so important to determine the presence of antibodies to hepatitis in time.
What methods are used to determine viral load?
- Polymerase chain reaction (PCR).
It is the most popular research method that allows you to detect even small amounts of viral RNA. This analysis has only one drawback - it is too expensive. The essence of the test: the biomaterial under study is exposed to heat so that the viral RNA actively multiplies and the mass of the substrate under study increases. Next, a special dye is added that reacts with the RNA. Then, using a special scale, the degree of staining is assessed and a conclusion is drawn about the viral load. According to the purpose of the study, PCR can be qualitative, quantitative and genotyping. The sensitivity of the method is 50 IU in 1 ml and higher; - Branched DNA.
The method has several disadvantages - it is insensitive (from 500 IU in 1 ml), does not provide the possibility of genotyping, and often gives false negative results. But it also has advantages - low cost and the ability to test several samples at once. The branched DNA method is used to assess the quality of therapy in patients with an already established diagnosis. It is based on the use of two nucleotide probes. One end of the first probe is connected to a viral RNA molecule, and the other to an amplifier molecule. Next, a second probe is introduced: it is marked with a dye and its structure is similar to an amplifier molecule. The generated signal is recorded by chemiluminescence; - Transcriptional amplification (TMA).
The method has high sensitivity (5-10 IU/ml). Testing is carried out at a fairly low temperature. First, the RNA of the virus is subjected to an amplification procedure, then the result is recorded with a luminometer.
New drugs for treatment
As of November 2021, next-generation therapeutic regimens consisting of two to three replication inhibitors sofosbuvir/velpatasvir/voxilaprevir from Gilead and glezaprevir/pibrentasvir ± sofosbuvir, tested by AbbVie, are undergoing the final phase III of clinical trials. Both regimens demonstrate high pangenotypic activity and efficacy in multidrug-resistant individuals.
The first pangenotypic representatives of the class of non-nucleoside inhibitors of NS5B polymerase CC-31244 and the injectable form of a prolonged action GSK2878175 are undergoing phase I-II clinical trials. Both inhibitors can potentially be used in combination therapy with both other classes of DAAs and indirect-acting antiviral drugs.
Decoding viral load indicators
Normally, virus RNA is not detected in the blood. The table shows generally accepted estimates.
| Low load | High load |
| Less than 800,000 IU/ml | More than 800,000 IU/ml |
Test results for hepatitis C must be deciphered by a highly qualified specialist. It will take into account not only digital values, but also the dynamics of changes in viral load indicators. A low load will indicate a positive result of therapy.
How long can therapy last?
The choice of regimen and duration of treatment depends on the course and stage of hepatitis C, which is determined by the doctor. Treatment with a combination of interferon and ribavirin can last 12 months.
Moreover, unlike many other infectious diseases, for chronic hepatitis C there is no single standard of treatment; individual planning is recommended in special cases. Complex treatment protocols are provided, taking into account the genotype of the virus, the condition of the liver (indicators of its function and changes in its tissue during biopsy), and viral load.
Doses of drugs and the schedule for their administration may vary, and also depend on the types of drugs (for example, different forms of interferon).
How to reduce high viremia
After conducting an analysis for the quantitative determination of HCV and deciphering its results, the specialist develops treatment tactics:
- Direct-acting antiviral drugs
(“Maviret”, “Sofosbuvir”, “Daklatasvir”, “Vikeira Pak”, “Velpatasvir”, “Ledipasvir”, etc.). Medicines are effective, safe, have a small number of contraindications; - Combination “Interferon” + “Ribavirin”.
Today it is used quite rarely: its effectiveness is too low (if compared with the latest generation of medicines) and a large list of contraindications. However, if the patient cannot tolerate other antiviral drugs or has any restrictions, these drugs are prescribed to him; - Hepatoprotectors
(“Essentiale Forte”, “Hepa-Merz”, “Karsil”, “Legalon”, etc.). The drugs are prescribed to everyone who complains of liver problems. They should be taken for a long time until the liver recovers completely; - Multivitamin complexes.
These preparations contain B vitamins, antioxidants (vitamins A and E, ascorbic acid), basic macro- and microelements, and essential amino acids.
Properly selected complex therapy is one of the few ways to reduce the viral load. In addition, viremia can be quickly reduced with a strict diet. The patient should eat in accordance with table No. 5, if his condition is severe enough - with table No. 5a. Alcoholic drinks, drugs, and cigarettes are strictly prohibited.
Genotypes
Genotypes have a specific territorial distribution. For identical genotypes in different territories, the same principles of treatment apply. They are designated by Arabic numerals (from one to six), and quasitypes or subtypes are designated by letters of the Latin alphabet (a, b, c, d, e) and so on:
- First genotype. Distributed everywhere, three quasitypes are distinguished (1a, 1b, 1c). If this genotype is confirmed, long-term treatment should be expected, for one year or more.
- Second genotype. Characterized by the widespread distribution of the genotype and four quasitypes (2 a, b, c, d). The duration of treatment is usually no more than six months.
- Third genotype. Distributed everywhere. The presence of six quasitypes (3 a, b, c, d, e, f) has been proven. This genotype is characterized by fatty degeneration (infiltration) of the liver parenchyma - steatosis. Treatment time depends on the quality of diagnosis. The average treatment time is limited to six months.
- Fourth genotype. Distributed in the countries of the Middle East and Central Africa. In Russian conditions it is little studied. Ten quasitypes have been identified (4a, b, c, d, e, f, g, h, i, j).
- Fifth genotype. First registered in South Africa. Has one quasitype. In the conditions of our country it remains a little-studied pathology.
- Sixth genotype. Registered in Asian countries, has one quasitype. In Russian conditions it is little studied.
The term “genotype” means the differences of the virus at the molecular (genetic) level.
What to Follow Before Doing Viral Load Tests
- The test is taken on an empty stomach, you can only drink water;
- Two days before the study, alcohol, fatty and fried foods, strong coffee, tea should be excluded from the diet;
- 24 hours before the test, stop taking medications;
- Do not smoke an hour before the test;
- Minimize power loads;
- Refuse physiotherapeutic procedures and massage.
To make the results more accurate, tests should be taken in the same laboratory. Results obtained from different laboratories may vary. This will not allow the specialist to give an objective assessment of the patient’s condition, analyze the dynamics of the disease and decide on treatment tactics.
How much does treatment cost?
Costs for modern medications needed for treatment can range from $550 to $2,500 per month. The duration of treatment is 12 months (respectively, $6600-30000 per year).
Newer, effective, studied, easy-to-use drugs produced by well-known companies are more expensive - 40-100 thousand dollars for a course of therapy.
The main costs are for interferon drugs. Foreign-made pegylated interferons are more expensive than conventional interferons from any manufacturer.
Prevention
There is currently no specialized vaccine against hepatitis C. Therefore, to prevent the disease, it is recommended to follow a number of simple rules:
- When injecting, you cannot use one needle on several people.
- Piercing and tattooing instruments should be sterilized after each use, and the artist should use disposable gloves.
- Manicure tools, razors, toothbrushes must be personal and not used by other people.
- Safe sex. It must be remembered that although the likelihood of infection during unprotected sexual intercourse is relatively low, it increases sharply with casual relationships. In such cases, the use of a condom is mandatory.
To avoid infecting the unborn child, when planning a pregnancy, a woman should be tested for hepatitis C.
Anti-HCV, IgM
Anti-HCV, IgM - specific immunoglobulins of the IgM class to the proteins of the hepatitis C virus, indicating possible infection or reactivation of the process, and are a marker of the early stage of infection.
Synonyms Russian
Antibodies to hepatitis C virus, anti-HCV.
English synonyms
Antibodies to Hepatitis C Virus, IgM; HCVAb, IgM.
Research method
Enzyme-linked immunosorbent assay (ELISA).
What biomaterial can be used for research?
Venous blood.
How to properly prepare for research?
- Do not eat for 2-3 hours before the test; you can drink clean still water.
- Do not smoke for 30 minutes before the test.
General information about the study
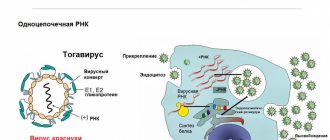
Hepatitis C virus (HCV) is an RNA virus from the Flaviviridae family that infects liver cells and causes hepatitis. It is able to multiply in blood cells (neutrophils, monocytes and macrophages, B-lymphocytes) and is associated with the development of cryoglobulinemia, Sjogren's disease and B-cell lymphoproliferative diseases. Among all the causative agents of viral hepatitis, HCV has the greatest number of variations, and due to its high mutational activity, it is able to evade the protective mechanisms of the human immune system. There are 6 genotypes and many subtypes of the virus, which have different implications for the prognosis of the disease and the effectiveness of antiviral therapy.
The main route of transmission of infection is through blood (during transfusion of blood and plasma elements, transplantation of donor organs, through non-sterile syringes, needles, instruments for tattooing, piercing). Transmission of the virus through sexual contact and from mother to child during childbirth is possible, but this occurs less frequently.
Acute viral hepatitis is usually asymptomatic and remains undetected in most cases. In only 15% of those infected, the disease is acute, with nausea, body aches, lack of appetite and weight loss, and is rarely accompanied by jaundice. 60-85% of infected people develop a chronic infection, which is 15 times higher than the frequency of chronicity with hepatitis B. Chronic viral hepatitis C occurs in waves with an increase in liver enzymes and mild symptoms. In 20-30% of patients, the disease leads to cirrhosis of the liver, increasing the risk of developing liver failure and hepatocellular carcinoma.
Specific immunoglobulins are produced for the core (nucleocapsid protein core), shell (nucleoproteins E1-E2) and fragments of the virus genome (NS non-structural proteins). In most patients with HCV, the first antibodies appear 1-3 months after infection, but sometimes they can be absent from the blood for more than a year. In 5% of cases, antibodies to the virus are never detected. In this case, HCV will be indicated by the detection of antibodies to virus antigens.
During the acute period of the disease, antibodies of the IgM and IgG classes are formed to the nucleocapsid protein core. During the latent course of the infection and during its reactivation, IgG antibodies to the non-structural proteins NS and the nucleocapsid core protein are present in the blood.
IgM to HCV begins to be produced 4-6 weeks after infection and quickly reaches a maximum. After 5-6 months, their level drops and rises again during reactivation of the infection. The criteria for the acute phase are symptoms of acute hepatitis in the absence of indications of similar diseases in the past, increased levels of liver enzymes, detection of anti-HCV, IgM (with increasing titers during dynamic observation), detection of HCV RNA using PCR (carried out to clarify the activity of the process, the stage of the disease and treatment monitoring). The correlation between detection of RNA and antibodies (IgM) is 85-92%.
Long-term circulation of IgM antibodies (more than 2 months) indicates a possible chronicity of the process.
After an infection, specific immunoglobulins circulate in the blood for 8-10 years with a gradual decrease in concentration or remain for life in very low titers. They do not protect against viral infection and do not reduce the risk of re-infection and development of the disease.
What is the research used for?
- To diagnose viral hepatitis C, clarify the activity of the process, the stage of the disease.
- For differential diagnosis of hepatitis.
When is the study scheduled?
- For symptoms of viral hepatitis and increased levels of liver transaminases.
- If it is known that you have had hepatitis of unspecified etiology.
- When examining people at risk for infection with viral hepatitis C.
- During screening examinations.
What do the results mean?
Reference values: not found.
Reasons for the positive result:
- acute viral hepatitis C.
Reasons for negative results:
- absence of hepatitis C virus in the body;
- low level of antibodies - early period after infection;
- absence of antibodies in viral hepatitis C (seronegative variant, about 5% of cases).
What can influence the result?
- Rheumatoid factor in the blood contributes to a false positive result.
Important Notes
- If the result is positive, to confirm the diagnosis of hepatitis C virus, a test is performed to determine the structural and non-structural proteins of the virus (NS, Core).
- If there are existing risk factors for infection and suspicion of viral hepatitis C, it is recommended to determine the RNA of the virus in the blood by PCR, even in the absence of specific antibodies.
Also recommended
- Anti-HCV, antibodies
- Antibodies to structural and non-structural proteins of the hepatitis C virus
- HCV, RNA [real-time PCR]
- HCV, genotyping (types 1a, 1b, 2, 3a, 4), RNA [real-time PCR]
- anti-HAV, IgM
- HBsAg, hypersensitive
- Gamma-glutamyl transpeptidase (gamma-GT)
- Alanine aminotransferase (ALT)
- Aspartate aminotransferase (AST)
- Total alkaline phosphatase
- Total bilirubin
Who orders the study?
Infectious disease specialist, hepatologist, gastroenterologist, therapist.
Literature
- Harrison's Principles of Internal Medicine. 16th ed. NY: McGraw-Hill; 2005: 1822-1855.
- Lerat H, Rumin S, Habersetzer F, and others. In vivo tropism of hepatitis C virus genomic sequences in hematopoietic cells: influence of viral load, viral genotype, and cell phenotype. Blood. 1998 May 15;91(10):3841-9.PMID:9573022.
- Revie D, Salahuddin SZ. Human cell types important for hepatitis C virus replication in vivo and in vitro: old assertions and current evidence. Virol J 2011 Jul 11;8:346. doi:10.1186/1743-422X-8-346. PMID:21745397.