Study of the biocenosis of the urogenital tract in women (Femoflor-16)
Femoflor is a unique technology based on the use of polymerase chain reaction (PCR) in real time. This technology today allows us to give the most complete quantitative and qualitative characteristics of the normal and opportunistic flora of the urogenital tract in women. Femoflor makes it possible to study difficult-to-cultivate anaerobic microorganisms and at the same time has high sensitivity and specificity.
What is biocenosis?
Biocenosis (from the Greek βίος - “life” and κοινός - “general”) is a collection of different types of microorganisms interconnected by certain relationships and inhabiting a certain biological niche. In the case of vaginal biocenosis, the biological niche for microorganisms is the vagina. The vaginal microflora in women of childbearing age is a well-balanced, stable system and can include more than a hundred species of different bacterial microorganisms, the main of which are lactobacilli, which constitute the normal microflora. Lactobacilli produce hydrogen peroxide and lactic acid, which reduces the vaginal pH to 4.0-4.5. The acid reaction is favorable for the growth and reproduction of lactobacilli, but not other microorganisms. It is known that lactic acid bacteria stimulate local immunity. Due to these factors, lactobacilli protect the woman’s genitourinary system, prevent the colonization of the vagina by pathogenic microorganisms and the excessive proliferation of opportunistic bacteria. In addition to lactobacilli, other microorganisms (gardnerella, yeast-like fungi, streptococci, staphylococci and others) may be present in the vagina in small quantities. The composition of microorganisms inhabiting the female body, including the genitourinary system, is unique for each woman and depends not only on age, but also on lifestyle. Knowing the characteristics of the composition of microorganisms, you can notice even small deviations in time and, even before the appearance of unpleasant symptoms, correct them with minimal effort, preventing the development of the disease.
It is important that the vaginal biocenosis is involved in ensuring a woman’s reproductive function, protecting her from possible infectious processes.

An imbalance between normal microflora and opportunistic microorganisms leads to the development of dysbiosis. Dysbiotic disorders cause a number of problems - from minor discomfort, a decrease in a woman’s quality of life to serious reproductive problems. There are many reasons for the development of dysbiotic processes in the vagina:
- changes and disturbances in hormonal levels (irregular sex life, pregnancy, childbirth, abortion, any type of cycle disorders, puberty, menopause, use of hormonal contraceptives and medications);
- use of intrauterine devices;
- promiscuous sex life (neglect of barrier contraception, frequent change of sexual partner);
- infections of the genital organs (infectious and inflammatory diseases of the pelvic organs, sexually transmitted infections);
- taking antibiotics (long-term or repeated courses);
- intestinal diseases (chronic problems with stool, intestinal dysbiosis);
- violation of personal hygiene rules (improper use of tampons creates favorable conditions for the growth of bacteria, frequent douching, use of cosmetic detergents for intimate hygiene, wearing tight synthetic underwear);
- decreased general and local immunity;
- hypothermia of the body; unbalanced diet (lack of vitamins and microelements);
- stress and overwork;
- change of climate zone.

Why is the research being conducted?
The study is carried out to determine the presence, degree and nature of microflora imbalance. Which, in turn, is extremely important for the clinician when choosing treatment tactics and for monitoring the effectiveness of the treatment.
To whom and when is this study indicated?
This study is carried out:
- if you are bothered by unpleasant symptoms (itching, burning, discharge, discomfort in the genital area);
- before planned surgical intervention in the pelvic area;
- if there is a feeling of discomfort after taking hormonal drugs, antibiotics;
- infertility;
- miscarriage;
- pregnancy planning;
- preparation for in vitro fertilization
- with ineffective treatment, with unclear results of other laboratory diagnostic methods.
It is important to understand that microflora imbalances that affect your quality of life are not only inconvenient, but can also lead to problems. Timely diagnosis will help identify even minor violations. Therefore, if you experience the slightest discomfort, you don’t have to wait long and don’t put off visiting a doctor.
Study of vaginal microbiocenosis with determination of sensitivity to antibiotics
Bacteriological examination of material obtained from the vagina, which allows you to assess the quantitative composition of the microflora, the ratio of microorganisms, identify a decrease in the number of lactobacilli, an increase in the growth of facultative microorganisms or the appearance of atypical microorganisms and determine their sensitivity to antibacterial drugs.
Synonyms Russian
Culture for vaginal dysbiosis with sensitivity to a/b, culture of a vaginal smear on a tank. biota and sensitivity to a/b.
English synonyms
Vaginal Culture with Antibiotic susceptibility testing.
Research method
Microbiological method.
What biomaterial can be used for research?
Urogenital smear.
How to properly prepare for research?
- Women are recommended to take a urogenital smear before menstruation or 2-3 days after it ends.
General information about the study
The normal microflora of the vagina, due to the stability of its quantitative and species composition, prevents the colonization of the vagina by pathogenic microorganisms and suppresses the excessive proliferation of opportunistic pathogenic microorganisms (OPM), which are included in small quantities in the normal microcenosis. The vaginal microecosystem consists of permanently inhabiting (obligate) and transient (occasional) microorganisms. The main representatives of the normal vaginal microflora are:
- gram-positive obligate anaerobic and microaerobic bacteria (lactobacteria, bifidobacteria, peptostreptococci, clostridia, propionobacteria, mobiluncus),
- gram-negative obligate anaerobic bacteria (bacterioides, prevotella, porphyromonas, fusobacteria, veillonella),
- facultative anaerobic microorganisms (gardnerella, corynebacteria, mycoplasma, staphylo- and streptococci, enterobacteria, yeast, fungi of the genus Candida).
At different periods of a woman’s life, depending on the activity of the reproductive function and hormonal balance, the vaginal microcenosis has certain characteristics. Before the first menstruation and the formation of menstrual function against the background of non-functioning ovaries, the microflora of the vagina is dominated by gram-positive cocci - epidermal and other coagulase-negative staphylococci, micrococci, non-hemolytic streptococci. Non-pathogenic Neisseria and Corynebacteria are less common, and Escherichia and Enterococcus are even less common. As puberty progresses, the number of lactobacilli increases, and in sexually mature girls the microflora is almost entirely represented by lactobacilli.
In healthy women of reproductive age, the total number of microorganisms in vaginal discharge is 107-109 CFU/ml (colony forming units per milliliter) and consists of more than 40 different species. The predominant species are Doderlein bacilli – Lactobacillus spp. (95-98%) - a large group of bacteria, mainly microaerophiles. Despite the diversity of the species composition of lactobacilli isolated from the vagina of healthy women (more than 10 species), it is not possible to identify a single species that would be present in all women. Most often it is possible to isolate the following lactobacilli: L. acidophilus, L. brevis, L. jensenii, L. casei, L. leishmanii, L. Plantarum. Among transient vaginal microorganisms, the most common are coagulase-negative staphylococci, primarily S. epidermidis, Corynebacterium spp., Streptococcus spp., Bacteroides, Prevotella spp., Mycoplasma hominis, which are usually present in moderate quantities (up to 104 CFU/g). Micrococcus spp., Propionibacterium spp., Veillonella spp., Eubacterium spp. are found just as often, but in smaller numbers. Among the relatively rare microorganisms (less than 10% of those examined) are Clostridium spp., Bifidobacterium spp., Actinomyces spp., Fusobacterium spp., Ureaplasma urealyticum, Staphylococcus aureus, Neisseria spp., E. coli and other coliform bacteria, Mycoplasma fermentans , Gardnerella vaginalis, Candida spp.
A decrease in the number of lactobacilli and excessive growth of opportunistic microorganisms leads to dysbiotic disorders, which can clinically manifest as inflammation of the vaginal walls - vaginitis, accompanied by severe itching, burning, and abnormal discharge. Pathological changes in the vaginal microcenosis can occur during treatment with antibiotics (local or systemic), cytostatics, hormones, radiation therapy, especially against the background of endocrinopathies (primarily diabetes), with anemia, congenital malformations of the genital organs, with the use of contraceptives and disorders in the immune system.
A study of vaginal microbiocenosis (bacteria culture) helps to diagnose dysbiosis (bacterial vaginosis), identify the causative agent of nonspecific bacterial vaginitis, fungal infection, pelvic inflammatory disease or sexually transmitted infections.
This research method is based on the ability of microorganisms to multiply on artificial nutrient media, which makes it possible to identify the types of bacteria and fungi living on the mucous membrane, determine their concentration and sensitivity to antibacterial drugs. When a probable causative agent of an infectious-inflammatory process is identified, it is incubated with antibiotics or diffusion disks impregnated with a drug are used - a drug to which the isolated microorganism is sensitive - this prevents the growth of bacteria.
The bacteriological method is indispensable for infections caused by opportunistic microorganisms (urogenital mycoplasmas, yeast fungi, Entrobacteriaceae, Streptococcus spp., Staphylococcus spp., etc.), since only with its help can the amount of the pathogen be assessed. Determining sensitivity to antibacterial drugs allows you to avoid the prescription of useless but unsafe drugs and select adequate therapy.
What is the research used for?
- For the diagnosis of dysbiotic disorders of the vaginal microflora;
- to identify the microorganism that caused the development of the infectious and inflammatory process of the vagina and pelvic organs;
- to determine drugs to which the causative agent of the infectious-inflammatory process is sensitive (to select effective antibacterial therapy);
- for the diagnosis of nonspecific bacterial vaginitis/vulvovaginitis, bacterial vaginosis, vulvovaginal candidiasis.
When is the study scheduled?
- With clinical signs of inflammatory diseases of the vagina and pelvic organs (itching, burning, leucorrhoea);
- when infectious and inflammatory changes are detected by microscopy of a vaginal smear;
- if treatment for vaginitis is ineffective;
- when selecting antibacterial drugs for the treatment of inflammatory diseases.
What do the results mean?
The result is interpreted by the attending physician taking into account the patient’s complaints, medical history, clinical manifestations of the disease and the exclusion of sexually transmitted infections.
Sensitivity to antibiotics is determined by identifying diagnostically significant growth of opportunistic biota.
Normally, lactobacilli predominate in the culture; opportunistic microorganisms are absent or detected in small quantities - less than 104.
With bacterial vaginosis, the growth of lactobacilli is sharply reduced or absent, and the number of opportunistic microorganisms is increased. When cultured, anaerobic microorganisms, Gardnerella vaginalis, Mycoplasma hominis, Mobiluncus spp., Bacteroides spp., peptostreptococci, can be isolated.
With vulvovaginal candidiasis, against the background of a decrease in the number of lactobacilli, an increase in Candida spp is observed. more than 104. With this pathology, microscopy of the material should reveal pseudomycelium of the fungus.
Nonspecific bacterial vulvovaginitis is characterized by the growth of one or more opportunistic microorganisms in a diagnostically significant titer with a decrease in the number or absence of lactobacilli in the culture.
Important Notes
- It is recommended to combine the study with microscopy of genital discharge, Gram-stained. If there are clinical signs of infectious and inflammatory diseases of the genital organs, it is necessary first of all to exclude sexually transmitted infections.
- It should be remembered that all opportunistic microorganisms can also be found in healthy women and exhibit their pathogenic properties only at significant concentrations. The results of the study should be interpreted taking into account the symptoms of the disease and the patient’s complaints.
Also recommended
- Microscopic examination of discharge from the genitourinary organs of women (microflora), 3 localizations
- Analysis of vaginal microbiocenosis. 16 indicators, DNA quantitative [real-time PCR]
- Analysis of vaginal microbiocenosis. 8 indicators, DNA quantitative [real-time PCR]
- Culture for Gardnerella vaginalis with determination of titer and sensitivity to antimicrobial drugs
- Neisseria gonorrhoeae, DNA [real-time PCR]
- Trichomonas vaginalis, DNA [real-time PCR]
- Ureaplasma species, DNA quantitative [real-time PCR]
- Culture for Ureaplasma urealyticum with determination of sensitivity to antibiotics (with a titer of 1x10^4 and higher)
- Chlamydia trachomatis, DNA [real-time PCR]
- Culture for Chlamydia trachomatis with determination of sensitivity to antibiotics
Who orders the study?
Obstetrician-gynecologist.
Literature
- Daniels R. Delmar's Guide to Laboratory and Diagnostics Tests // Cengage Learning – 2009 – 1003 p.
- Larsen B., Monif G. Understanding the bacterial flora of the female genital tract. // Clin Infect Dis. – 2001 – 32 (4) – p.69-77.
- Infections in obstetrics and gynecology / Ed. O. V. Makarova, V. A. Aleshkina, T. N. Savchenko. – M.: MEDpress-inform, 2007. – 464 p.
Vaginal biocenosis and antimicrobial therapy.
- Korzhuev S.I.
- Nov 08, 2012
- For doctors
- Comments on the entry Vaginal biocenosis and antimicrobial therapy. disabled
What's happening
In 1996, a commission of the Ministry of Health of the Russian Federation stated the fact of the epidemic spread of sexually transmitted infections. And since then, the incidence has only continued to rise. In many ways, this situation can be explained by the early onset of sexual activity, the lack of basic knowledge about safe sex and intimate hygiene, a certain freedom of sexual relations and numerous casual and spontaneous sexual contacts.
The number of women with sexually transmitted infections (gonorrhea, trichomoniasis, chlamydia, syphilis, herpes, HIV infection, hepatitis C virus) has now increased 4 times compared to the generation of their mothers. And against this background, infectious diseases of the genital area are increasingly arising as a result of the activation of opportunistic infections.
Measures that are so effective for classical infections turned out to be of little use for the treatment of diseases caused by opportunistic microflora. And today, opportunistic bacteria (Klebsiella, Proteus, Escherichia coli, enterococci, multidrug-resistant strains of opportunistic bacteria - obligate anaerobes, microbial associations) constitute obvious competition for pathogens of sexually transmitted diseases. The spectrum of infections has become dominated by diseases of the genital organs that develop against the background of dysbiosis.
What is the reason
Such shifts seem unexpected only at first glance. The predictability of this situation lies in the lack of health improvement among adolescents (in 63% of them, early sexual debut is accompanied by defloration cystitis), in the high frequency of chronic general somatic diseases (impairing immunoresistance), most of which remain unrecognized by the time pregnancy occurs.
Abortion makes its contribution - the only free method of birth control in the compulsory medical insurance system, unnatural in its essence, breaking all the anti-infective barriers given to women by nature. The culmination of the destruction of natural microflora in the absence of periabort sanitation becomes aseptic or purulent (almost 15%!) endomyometritis.
What are the consequences
The consequences of preconceptional lactobacilli deficiency after gynecological inflammatory diseases and surgical interventions are expected and sad. The desired pregnancy may not occur in the future. And if such a miracle occurs, then the risk of complications is extremely high - a non-developing pregnancy, its spontaneous termination, a long-term threat and the birth of a low-weight child infected in utero. After birth, such a child’s somatic status is disrupted and the quality of life decreases.
If we imagine these losses on a national scale, then it is no wonder that we are on the verge of a demographic catastrophe - the low birth rate is complemented by a high morbidity and morbidity index.
And it all starts with the fact that young women do not want, and doctors do not insist on banal pre-gravid preparation and do not correct the biocenosis during pregnancy.
General plan for biocenosis correction
Correction of vaginal microflora in the pre-conception period is unthinkable without observing the basic postulates - eliminating glycogen deficiency (a nutrient medium for lactobacilli), i.e. restoration of a two-phase menstrual cycle, as well as restoration of a full perineum.
In general, the complex of means for restoring normobiocenosis includes the following points.
- Elimination of pathogenic bacteria.
- Restoration of the anatomical and functional usefulness of the perineum.
- Elimination of constipation (which ensures a 3-fold increase in lactobacilli in the vagina).
- Diet: sauerkraut, yogurt, biokefirs.
- Eubiotics, restoration of the acidity of the vaginal environment with ascorbic acid.
- Hormonal regulation of the menstrual cycle with the help of COCs and gestagens.
- Phytoestrogens
The most important condition for correcting any form of dysbiosis should be considered the restoration of the normal architecture of the perineum, vagina, and cervix: elimination of the “gaping” genital cleft after childbirth.
Unfortunately, the attitude of patients, as well as doctors, to this issue is extremely frivolous, contrary to the guidelines of modern reproductive medicine: the flow of vaginal secretion makes maintaining normal biocenosis impossible.
Correction of estrogen deficiency conditions is necessary to maintain a certain estrogen-progesterone gradient, since it is thanks to a sufficient amount of estrogens that the required level of vaginal glycogen is achieved, the breakdown of which by lactobacilli to lactic acid and hydrogen peroxide ensures the death of up to 90% of infections.
From these positions, the use of not only natural estrogen-progestin drugs - Femoston, modern COCs, but also phytoestrogens (soy, black cohosh and red clover) is justified.
An ingenious way is to “acidify” the vaginal environment with ordinary ascorbic acid in the form of vaginal tablets (Vaginorm-S). This leads to an obstacle to the proliferation of anaerobic bacteria and the growth of lactobacilli to actual normobiocenosis.
"Treatment tests"
There are two subjective reasons for the increase in dysbiotic and infectious-inflammatory diseases of the genitals:
- irrational, often unfounded antimicrobial treatment of non-existent diseases - due to incorrect interpretation of laboratory test results by doctors, in particular high-quality PCR, as well as
- self-medication with various over-the-counter and even prescription drugs with antimicrobial action.
Unfortunately, we often treat what should not be treated, and vice versa. This happens for quite ordinary reasons: we are accustomed to trusting tests, of which, if common sense is not followed, a pregnant woman is prescribed a huge number. The information content of high-quality PCR, the most popular test in antenatal clinics, is clearly overestimated. The result of this expensive method is the detection of infection in any titer, including less than it has the right to be normal.
Diagnosis of urogenital infections using qualitative PCR in Russia is performed 6 times more often than in the USA. That is why there are numerous so-called “-oses” (mycoplasmosis, ureaplasmosis, gardnerellosis) - an exclusively domestic “product” (they are not listed as such in ICD-10!). In the USA, in order to stop the desire to “treat tests”, and also because of the “high cost and over-informativeness”, from January 1, 2007, it was considered inappropriate to prescribe even a bacterioscopic (!) examination of the discharge of pregnant women without complaints of pathological leucorrhoea.
It is amazing that with all the desire to “treat for good,” repeated screening for infections after the so-called “sanitation” is carried out in only 4% of patients! Of the “treated” women, 96% are not subjected to a follow-up examination and, therefore, no restoration of vaginal normocenosis is carried out.
Irrational antibiotic therapy
Antibiotic therapy, which has become so common in identifying any symbionts only recently and far from everywhere, has finally begun to be analyzed from the point of view of the consequences of these so thoughtless “aggressive” medical influences.
The global consequences of the vicious tactics “more tests → more treatment → distortion of the natural biocenosis” confirms the conclusion made at the G8 summit, at the satellite symposium of health ministers in St. Petersburg in 2007.
By mutual agreement of the ministers, $2 billion was allocated to create a vaccine against pneumonia. Thus, all other ways and attempts to create new antibiotics against pneumococci were considered unpromising - too many of them were prescribed for indications and without, and as a result - absolute antibiotic resistance of pneumococci. In essence, this is the beginning of the end of the era of antibiotics.
Obstetricians and gynecologists are the absolute leaders in the frequency and senselessness of prescribing disinfectants and antibacterial agents.
What should be normal?
One of the reasons for the aggressive chemical attack that follows the absolutely unnecessary collection of numerous tests and is aimed at the sterility (!) of the vagina is outdated views on the biocenosis, the quantitative and qualitative composition of the vaginal microflora.
The dominance of lactobacilli, which were once the only representatives of the vaginal biotope, was replaced by bacterial diversity proclaimed by all European communities of obstetrics and gynecology: in every second healthy woman of reproductive age, gardnerella can be identified in the vaginal contents, in every fourth - Escherichia coli, in every fifth - mycoplasma. The presence of non-eubiotic bifidobacteria in the vaginal microflora is possible without the formation of vaginitis and vaginosis.
In the absence of clinical manifestations and bacterioscopically indicated suppuration, the presence of Candida is acceptable, which is the case in 25% of non-pregnant women and 50% of pregnant women. The existence of clostridia, corynebacteria, enterococci, mobiluncus, peptostreptococci, prevotella, E. coli is also possible if the CFU of these pathogens does not exceed 105, and the CFU of lactobacilli is more than 107, and there are no clinical signs of inflammation. The woman in this case is healthy and does not need any treatment.
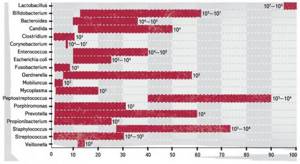
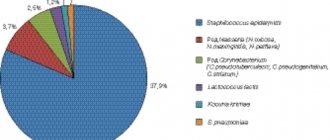
Frequency of isolation of microorganisms from the vagina of healthy women of reproductive age (%).
Quantitative studies of the vaginal microflora have shown: the total number of bacteria in the vagina in a normal vaginal ecosystem does not exceed 105-106 CFU/ml of discharge, while with bacterial vaginosis it increases to 109-1011 CFU/ml, with colpitis - up to 1012-1014 CFU/ml of discharge .
To treat or not to treat?
The reasons for the radical difference between modern vaginal microbiocenosis and that described in textbooks 15–20 years ago is a change in women’s lifestyle. Denim fashion, “denim” basins, tights instead of stockings, menstrual hygiene products (tampons, pads, especially daily ones) have led to disruption of aeration and an increase in the anaerobic component in microbial associations.
However, these changes should be approached from the standpoint of accepting this microbial community for granted, stopping unreasonable attempts to achieve complete sterility of the vagina. This is impossible in principle!
That is why reliable differential diagnosis of carriage, bacterial vaginosis and truly inflammatory diseases - vaginitis, cervicitis as realized infectious-inflammatory diseases - remains relevant in the problem of infections of the urogenital tract. The urgency of the question “To treat or not to treat?” highlights the increasing number of clinical trials and even lawsuits regarding inappropriate antibiotic therapy.
If you treat thoughtlessly
Practice shows that enhanced sanitation of the vagina is carried out against the background of even I-II degree of vaginal cleanliness, moreover, based on the results of uninformative tests - qualitative PCR and bacteriological research (“culture”) without determining quantitative characteristics.
Unreasonable treatment with disinfectants, powerful antibiotics, and without determining the sensitivity of the microflora, is carried out in 90% of women examined for urogenital infection using a qualitative PCR method.
The senselessness and unsafety of such “treatment” is confirmed by information from one antenatal clinic, in which out of 440 pregnant women, 29% were diagnosed with one or another urogenital infection in the first trimester. Paradoxically, the titer of lactobacilli in 24% of women who refused antibacterial therapy for various reasons (difficult financial situation, ideological beliefs) turned out to be higher in comparison with the cohort treated according to standard regimens.
Perversion of biocenosis. Medical activity in the absence of clinical manifestations in the patient inevitably leads to the destruction of all microflora and the creation of an “empty space” not intended by nature, soon populated by the same or similar microbes, but with increased antibacterial resistance, i.e. in fact, to the distortion of the natural biocenosis.
The realization of the negative consequences of the use of chlorhexidine occurs after 2-3 hours: in 95%, it is not a “sterile” result, but an opportunistic microflora, in 50% it is more pathogenic than the original one. The aggressiveness of traditional sanitation was demonstrated by the frequency of vaginal candidiasis - in the “treated” it was 2 times more common.
Birth trauma. In dissonance with the stable ideas of the relationship between ruptures of the perineum and cervix with a large head and high perineum, it is the condition of the tissue that determines the outcome - the severity of pelvic floor injuries correlates with the deterioration of the vaginal flora (Marilova N., 2007).
More than 100% of abnormal biocenoses were detected in the most traumatized women: III and IV degrees of vaginal cleanliness before the first birth were presented in 47.1 and 39.5%, respectively, and in 15.9% candidiasis was clinically diagnosed. The lower incidence of rupture corresponded to a limited number of cases of vaginitis (13.2%).
The conclusion is simple - disruption of the vaginal biocenosis before childbirth, resulting in bacterial vaginosis and vaginitis, increases the risk of perineal injury - it ruptures only where there is a III-IV degree of cleanliness.
Unfortunately, the desire for small incisions when performing episio- and perineotomy leads to further continuation of the wound, but in the form of a laceration - in 63% of cases. But this terrible figure also increases by 20% in conditions of a perverted, usually iatrogenic, biocenosis.
Continuing the vicious circle, the lack of surgical correction of prolapses predetermines the impossibility of restoring normobiocenosis, which, as a rule, is the case in most cases.
Postpartum infectious complications. The terrible essence of the vicious circle generated by the prescription of antibiotics for carriage and bacterial vaginosis without subsequent restoration of the microflora is the increase not only in postpartum injuries, but also in postpartum infectious diseases.
The occurrence of postpartum endometritis and uterine subinvolution as a veiled form of endometritis is 2 and 1.5 times more often recorded against the background of aggressive antibiotic therapy. In untreated patients, the incidence of postpartum purulent-septic diseases did not exceed that in the population.
Summarizing information about attempts to achieve sterility not only in the vagina, but also in the maternity hospital, it should be noted critically: in accordance with the order of the State Sanitary and Epidemiological Supervision of 2000, we have the right to the existence of hospital infections. At the same time, the main condition for the effectiveness of combating it is knowledge and monitoring of infections, infection control.
Maternal mortality. The consequence of an unjustified “chemical” attack on the vaginal microflora is not only the formation of a very difficult to treat condition, but also an extremely serious issue associated with the analysis of maternal mortality rates (2007).
Thus, 2/3 of those who died from abortion (13.2%) + 13.4% from postpartum sepsis (in total 26.6%) constitute the absolute first place in the structure of maternal mortality. Antibiotic therapy becomes ineffective; immunoglobulins improve outcomes, but do not solve the problem.
Iatrogenic dysbiosis should be considered as one of the controllable causes of maternal mortality from sepsis.
Rational diagnosis
The logical way to solve this problem would be to diagnose the vaginal microflora: at present one should focus on bacterioscopic data, the number of lactobacilli and, if everything is in order with this, if a healthy woman does not have III-IV degree of vaginal cleanliness, then no PCR- There is no need to subject it to research.
Qualitative PCR is informative only for the detection of microorganisms that should never be in the vagina (treponema pallidum, gonococci, chlamydia, trichomonas).
It should be emphasized that high-quality PCR does not replace bacteriological testing.
Here you need to pay attention to a number of nuances: up to 70% of trichomonas are not diagnosed using traditional methods, and it is rare that a laboratory doctor will take the liberty of making a diagnosis using a dry smear several hours later, when the flagella have fallen off. Only the hanging drop method is effective. Otherwise, the ability of Trichomonas to “preserve” gonococci and chlamydia (D.V. Ryumin, 2002) results in the development of “recurrent” urogenital infections and the need for an increasing number and dose of antibiotics and, accordingly, persistent mycotic damage.
With an “inflammatory” smear, the search for infection is carried out by quantitative PCR (the ratio of colonies of infection and lactobacilli) using a diagnostic multiprimer kit to determine the nucleotide sequences of Lactobacillus spp. (558 n.p.) and Gardnerella vaginalis (820 n.p.) and bacteriologically (culture from the cervical canal for microflora and sensitivity to antibiotics).
If in doubt “to treat or not to treat,” we recommend adhering to the following medical tactics: if the CFU of these pathogens (E. coli, St. epidermidis, St. aureus, Streptococcus group B, Enterococcus faecalis, Enterobacter cloaceae, Klebsiella pneumoniae, Proteus mirabilis) does not exceed 105 , and the CFU of lactobacilli is more than 107 in the absence of clinical manifestations of inflammation, the woman is considered healthy. It would be more logical to try to restore the biocenosis.
Trichomoniasis
According to current recommendations of the American Center for Disease Control and Prevention (CDC), metronidazole is the only drug of choice for the treatment of Trichomonas infection, which should be administered orally either 2 times a day or 500 mg 2 times a day for 7 days. These treatment regimens provide a cure rate of 90–95% of cases. However, in the first trimester of pregnancy during the period of organogenesis and placentation, treatment with procystocidal drugs is impossible. In the second trimester, the use of metronidazole and its derivatives in forms for topical use, for example in the form of vaginal suppositories, is permissible. The use of tablet forms of metronidazole and its derivatives is possible only in the third trimester.
Chlamydia infection
Despite the rather low expected probability of fetal infection with chlamydial infection (6–8%), with a transmission rate of 50–70%, timely and highly effective antibacterial therapy is necessary.
Among the antibiotics approved for the treatment of chlamydial infections during pregnancy (erythromycin, josamycin, rovamycin), the most attractive is vilprafen (josamycin), a wide spectrum of antimicrobial activity and low resistance of pathogens to which make it possible to cure mixed urogenital infections (chlamydia + mycoplasma) already in the first trimester. Many years of experience in use have shown its high effectiveness and harmlessness to the fetus at a dose of 500 mg 3-4 times a day for 10 days.
Myco- and ureaplasma infections
To the question whether or not to treat myco- and urea-plasma infections, we will answer with a quote from Prof. M.A. Bashmakova (2004): “I devoted half my life to myco- and ureaplasmas, and the rest I devote to ensuring that women are not treated without indications.”
Taking into account the fact that urogenital mycoplasmas are bacterial vaginosis-associated microflora, when the lower genital tract of pregnant women is colonized with M. hominis and/or U. Urealyticum, a routine set of diagnostic and, if necessary, therapeutic measures is recommended to restore normal vaginal microcenosis.
If indicated, according to a number of researchers, macrolides should be used as first-line drugs for mycoplasma infection. In the group of macrolides, josamycin has the lowest minimum inhibitory concentrations for mycoplasmas.
Due to its high activity and good tolerability, it is one of the most widely used antibiotics for the treatment of urogenital infections.
Josamycin has a wide spectrum of antibacterial action, including all clinically significant mycoplasmas (M. hominis, M. genitalium, U.
urealyticum), and chlamydia.
Bacterial vaginosis in the first trimester
Despite numerous studies on the correction of vaginal microbiocenosis, treatment of bacterial vaginosis during pregnancy remains a difficult problem due to the possible adverse effects of disinfectants on the fetus.
In the first trimester, the use of disinfectants is not recommended, moreover, it is dangerous, since they belong to risk categories C and D for the fetus. Although the effectiveness of treating bacterial vaginosis with the administration of clindamycin phosphate and metronidazole has been proven, their administration is safe only in the 2nd–3rd trimester of pregnancy (Azarova O.Yu. et al., 2002).
Povidone-iodine, which differs from all other drugs by its effect of preserving the acidic environment of the vaginal contents, can provide significant competition to oral drugs with antianaerobic activity. By preventing the growth of pathogenic and opportunistic microorganisms, the drug creates favorable conditions for the proliferation of lactobacilli. According to modern trends, it is possible to use dalacin cream or suppositories.
More recently, a new effective method of treating bacterial vaginosis has emerged - a special intravaginal form of ascorbic acid (“Vaginorm-S®”). A special feature of the drug is that by reducing the pH of the vagina to normal values, the growth of anaerobic microflora is suppressed and at the same time the growth of its own lactobacilli of the genus Lactobacillus is stimulated.
Restoration of biocenosis is an obligatory stage
It should be noted: after antibacterial therapy, which eliminates opportunistic microorganisms, conditions for a fairly rapid restoration of normal vaginal microflora are not created on their own.
Pregnancy occurring in conditions of abnormal biocenosis is unconditionally accompanied by a high risk of obstetric and perinatal complications. That is why it would be logical to complete the use of any justified “sanitation” of the vagina by restoring the pool of lactobacilli.
The fundamental difference between modern eubiotics is the base - not milk powder, as before, but fructooligosaccharides (apple pectin, carrot powder). Eubiotics created on dry milk, in the presence of mycelial threads, “curdled” and fell out of the vagina in the form of curdled discharge: not dangerous to health, aesthetically unacceptable.
Now the situation has changed radically: restoration of eubiosis by subsidizing lactobacilli can begin at the moment the use of disinfectants, antimycotics, etc. is completed. Therefore, during pregnancy, it is quite sufficient to carry out local treatment with the obligatory restoration of eubiosis with lactobacilli on a dairy-free, fructo-oligosaccharide basis (“Floraldophilus” is a symbiont of the last, fourth generation).
Due to the low adhesive properties of lactobacilli, achieving a pronounced acidophilic orientation of vaginal microorganisms is possible only if the following conditions are met: a sufficient number of bifidobacteria and lactobacilli in the intestines and the absence of constipation.
In 65–80%, the consequence of chronic constipation was a sharp decrease in the number of lacto- and bifid flora or its competitive replacement by strains of E. coli with reduced enzymatic or pathogenic properties, activation of opportunistic microflora (E. coli, Staphylococcus aureus and Staphylococcus epidermidis, etc.) [Gvasalia A.G., 2004]. With the elimination of constipation, the content of lactobacilli increases by 3 times.
In conclusion, it should be noted: the prevention of complications of pregnancy and childbirth requires very specific tactics - competent infectious screening and timely, adequate treatment for the identified pathogen. The main thing is to achieve a normal biocenosis with a lactobacilli content of at least 107 CFU/ml during pregnancy and at the end of childbirth, because the child should not contaminate E. coli and enterococcus, but the healthy lactoflora of a healthy mother.
Text: prof. V.E. Radzinsky, Ph.D. M.L. Pauline