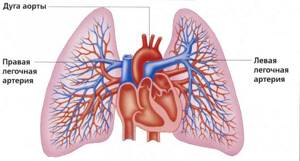
The physiology of the human body is based on the use of oxygen to produce energy. We get oxygen from the air. The result of gas exchange in the lungs is the combination of oxygen in the air with carbon (carbon dioxide), which the body no longer needs.
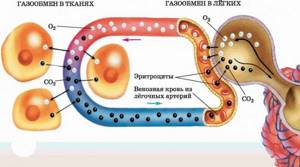
The transfer of oxygen into the blood (oxygenation), and the return of carbon dioxide with exhaled air into the atmosphere is called gas exchange. In order to evaluate the indicators of this process, the so-called blood gas analysis is used. There is no single or common test that is simply called “blood gas.” Among the indicators are the following values:
- partial pressure of oxygen;
- partial pressure of carbon dioxide.
The other two indicators are the concentration of bicarbonate, as well as the concentration of hydrogen protons, which is called pH or its acidity. In fact, blood pH is a dimensionless value, and it was specially created for the convenience of assessing the shift in the state of the electrolyte to the acidic or alkaline side.
What is partial pressure?
Not everyone understands well what partial pressure is. In simple terms, this is the pressure of a gas in a liquid at which the gas can already dissolve in it. It is the critical value of partial pressure that shows that the dissolved gas is well tolerated by the blood. If the gas is given complete freedom, it moves from an area of high pressure to an area of low pressure.
It is precisely this difference in partial potentials that exists in the lungs, where there is alveolar air on one side and capillary blood on the other. If the partial pressure of oxygen and carbon dioxide corresponds to normal values, then oxygen will unhinderedly saturate the blood, and carbon dioxide will perform the reverse process, leaving the blood and going into the atmospheric air. This process ends when the partial pressures of these gases become equal, or “balance the scales.” But the normal blood gas composition will never allow for equal pressures to be achieved; this is precisely what characterizes life.
If we recall a physics textbook, we can assume that since the oxygen content in the air is on average 21%, the partial pressure of oxygen in the air is 21% of atmospheric pressure, or 21 kilopascals (kPa), or 160 mmHg.
Content of gases in blood
The volume of gases in the blood in a state of physical dissolution can be determined using the formula given here.
Calculations show that in 100 ml of arterial blood the content of dissolved oxygen should be equal to 0.3 vol. %, carbon dioxide - 2.5 vol.% and nitrogen - 0.95 vol.%. However, much more oxygen and carbon dioxide can be extracted from the blood using the methods described. This indicates that oxygen and carbon dioxide are not only in a physically dissolved state, but also in a chemically bound state. Oxygen in the blood is bound to hemoglobin. Carbon dioxide is only partially bound to hemoglobin, but most of it is in the blood in the form of bicarbonate.
Extraction of gases from blood
. Gases were first completely extracted from the blood in 1859 by I.M. Sechenov, who designed a mercury pump for this purpose, based on the principle of a renewable Toricelli void.
In the Sechenov apparatus, a blood sample taken directly from a blood vessel is placed in a receiver, which is separated by a tap from a glass container. In the latter, a vacuum (Toricellian void) is created using a mercury pump and, by opening the tap, the balloon is connected to the receiver containing the blood. Gases immediately begin to be released from the blood (the blood seems to boil). The release of gases soon stops as equilibrium is established between the gases remaining in the blood and the gases that have passed into the cylinder. Then the tap is closed, the gases from the cylinder are transferred using the same mercury pump into a measuring vessel that serves to measure their quantity, and a Toricelli void is again created in the cylinder and the tap connecting the blood receiver to the cylinder is again opened. A new portion of gases passes from the blood into the balloon (until a new equilibrium is reached). By repeating this procedure several times, almost all gases can be extracted from the blood.
To extract gases from the blood, devices based on the principle of chemical displacement are also used. The most commonly used device is Barcroft's device, which measures the amount of oxygen displaced from the blood by adding a solution of potassium iron sulfide to it, and the amount of carbon dioxide displaced from the blood by adding tartaric acid.
The van Slyke apparatus is widely used, which combines the principles underlying the Sechenov and Barcroft devices. This device uses both the displacement of gases with chemical compounds and their extraction by creating a vacuum using a mercury pump.
Blood oxygen capacity
. Along with the study of blood content, the oxygen capacity of the blood is determined, i.e. the maximum amount of oxygen that can be absorbed by 100 ml of blood. To determine the oxygen capacity of blood, blood taken from a blood vessel is brought into contact with air so that it is completely saturated with oxygen.
The oxygen capacity of the blood depends on the hemoglobin content in it. Each gram of hemoglobin can bind 1.34 ml of oxygen. If the blood contains 14% hemoglobin, then 100 ml of blood can bind 14 × 1.34 ml of oxygen, i.e. 19 ml. This number (19 vol.%) is the normal oxygen capacity of the blood. Knowing the oxygen capacity of the blood and the oxygen content in the blood taken from the vessel and not in contact with air, it is possible to determine the degree of oxygen saturation of the blood, in other words, the ratio of the oxygen content in the blood under study to its oxygen capacity.
To determine the content of gases in the blood located in a blood vessel, blood taken with a syringe from the vessel is released under a layer of petroleum jelly or ammonia so that it, without coming into contact with air, retains the amount of gas that was in it.
Gas content in arterial and venous blood . The arterial blood of a healthy person contains 18-20 vol.% oxygen, 50-52 vol.% carbon dioxide and about 1 vol.% nitrogen. Venous blood contains 12 vol.% oxygen, 55-57 vol. % carbon dioxide and about 1 vol. % nitrogen. From these figures it follows that venous blood, passing through the pulmonary capillaries, is enriched with oxygen and gives up part of the carbon dioxide it contains. Arterial blood, entering the capillaries of the systemic circle, gives up part of its oxygen and is saturated with carbon dioxide. The same nitrogen content in arterial and venous blood indicates that it does not participate in gas exchange.
Uneven gas exchange in the lungs
If the normal blood gases in the patient’s body are disturbed and there are any disorders of alveolar ventilation, then the partial pressure of oxygen drops and that of carbon dioxide increases. Why is this happening?
The fact is that in the lungs the blood capillaries are not all very well adjacent to the alveoli; there are also those that are less well ventilated. As a result, the so-called ventilation-perfusion ratio changes and the blood, having passed through such a poorly ventilated alveolus, returns to the arterial bed with less oxygen and more carbon dioxide. This phenomenon is widely known and is called shunting, or reset. The body fights this by hyperventilation, that is, by increasing gas exchange in well-ventilated alveoli.
As a result, more carbon dioxide is exhaled and we inhale the amount of oxygen we need. These and similar disorders will be revealed by a blood gas test.
A little about ventilation and gas exchange disorders
Since our body vitally needs oxygen, the universal and basic concept of gas exchange disturbance is hypoxia. Literally, this is the name for any condition in which organs and tissues receive little oxygen, and this amount cannot provide normal aerobic (using oxygen from the air) metabolism.
However, there are two more important concepts related to hypoxia:
Hypoxemia. If hypoxia refers to capillary circulation in tissues, then hypoxemia is a deficiency of dissolved oxygen in red, arterial blood.
There can be many reasons: lung pathology, low amount of hemoglobin due to anemia, carbon monoxide poisoning;
Impaired oxygenation, or a special form of hypoxemia, in which the transport of oxygen from the lungs to the blood is reduced. The result is a low partial pressure of oxygen in the blood, less than 80 mmHg, or 10.7 kilopascals.
But the patient may develop gas exchange deficiency not only with a low partial pressure of oxygen in the blood, but also against the background of a high partial pressure of carbon dioxide, which will dominate and will not allow oxygen to pass into the tissues in the capillaries. This condition is called hypercapnia. This will also be noticeable when performing a blood gas test.
Finally, in clinical practice, hemoglobin oxygen saturation is also determined, that is, the percentage of hemoglobin that is associated with oxygen. It measures how well the blood can carry oxygen. Typically, a healthy person breathing normal atmospheric air has an oxygen saturation above 96%, and this is normal.
Currently, there are devices called pulse oximeters, and they are capable of measuring saturation indicators without contact. This device is worn like a clothespin on your finger, and the bedside monitor shows oxygen saturation in real time. When it decreases, an alarm is sounded.
Taking a blood gas test allows you to identify these and other types of gas exchange deficiency, make a diagnosis and prescribe treatment. What conditions are indications for taking a blood gas test?
Studies of electrolytes and blood gases. Preanalytical stage.
Preanalytical stageAcid-base state of the body
The acid-base balance of the body (acid-base balance, acid-base state, ABC) is the ratio of the concentrations of hydrogen ions H+ and hydroxyl groups OH in the biological fluids of the body.
The vital activity of the body is associated with the processes of tissue respiration: the body needs a sufficient amount of oxygen and the removal of excess carbon dioxide formed as a result of numerous metabolic reactions. Basic acid is a continuous process of formation and release of acids, which, under conditions of undisturbed metabolism, are released into the external environment: CO2 - by the lungs, heavy acids - by the kidneys.
Excessive accumulation of acids leads to acidosis, excessive secretion of acids leads to alkalosis - life-threatening conditions that require rapid, accurate diagnosis and rapid targeted treatment.
Changes in acid-base balance indicators indicate disturbances in gas exchange and metabolic processes. By assessing acid-base balance, one can judge the severity of the developing pathology, the dynamics of the patient’s condition and the adequacy of therapy.
Clinical laboratory diagnostics of acid-base status
Analysis of acid-base balance belongs to the category of express diagnostics and is performed in departments where patients are in critical condition (intensive care units, operating rooms). In these conditions, acid-basic acid analysis must be carried out urgently; procedures that determine the life or death of the patient depend on its results. The total time for issuing acid base analysis results for a number of indicators should not exceed 5-15 minutes.
Modern analyzers successfully cope with the task of obtaining accurate results from studying blood gases, electrolytes, oximetry parameters, and metabolites as quickly as possible. But even the most modern laboratory equipment, which analyzes a sample in the measuring channel with a high degree of accuracy, is hostage to the quality of this sample, which, in turn, is determined at the preanalytical stage. The choice of sample type and method of collection, and proper storage have a decisive influence on the test results.
ACB parameters can be determined in whole blood of any type - arterial, capillary, venous.
Arterial blood is the recommended type of sample for the analysis of blood gases and electrolytes: it is saturated with oxygen, its gas composition and metabolic parameters are the most stable.
Venous blood contains products of tissue metabolism; its gas composition is less constant, depends on peripheral blood flow and does not provide “representativeness” of the whole organism. Venous values of pO2 (partial pressure of oxygen) and sO2 (oxygen saturation) do not provide the necessary diagnostic information about oxygen transport and absorption.
Capillary blood is mixed; intensive metabolism occurs in the capillaries, so it is very difficult to obtain reliable information about acid-base balance at a given time. pO2 and sO2 values may not be reliable because the oxygen status of capillary blood is different from that of arterial blood. Capillary blood may be an alternative sample choice for acid-basic acid analysis if it is difficult to obtain blood from an artery.
The minimum volume of blood required for the analysis of blood gases and electrolytes is 200 μl, when sampling with a syringe or capillary.
The time interval between sample collection and blood gas and electrolyte testing should be minimal. Whole blood is a living tissue; active metabolism continues even in the sample that has already been taken. Leukocytes, platelets, reticulocytes consume oxygen and glucose, release CO2 and H+; red blood cells release lactate and H+ (anaerobic glycolysis). If the sample cannot be examined within 5 minutes of collection, it must be quickly cooled to +4°C in an ice bath. Under such conditions, metabolic processes in the sample slow down and, accordingly, the values of the acid-basic acid parameters at the time of collection are preserved. The sample remains suitable for research within 60 minutes. However, storing in an ice bath increases the risk of hemolysis and cannot be used for plastic syringes because it increases the permeability of the plastic to gases.
The blood clotting process begins immediately after taking a sample: microscopic clots form within 15 seconds and can lead to sample inhomogeneity and incorrect measurement results for some parameters (pCO2, pH, Hb). Clots can interfere with the operation of the device and also lead to incorrect results, without any indication of their error, which can affect the treatment of the patient. A complete blockage of the system may also occur, making it impossible to take further measurements until the fault is corrected.
Heparin is the only anticoagulant recommended for acid-basic acid analysis. However, it is important to remember that regular heparin, due to its binding effect, causes abnormal electrolyte measurements, especially ionized calcium. Conventional types of heparin (lithium and sodium) have free negative zones, with which the positive plasma ions Ca++, K+ and Na+ are connected. Heparin-bound ions cannot be detected by ion-selective electrodes, and the device will produce a result lower than the actual value. For blood gas analysis, balanced heparin should be used, which contains electrolytes in quantities identical to normal plasma, which significantly reduces the binding of electrolytes and increases the accuracy of the results.
Electrolyte-balanced heparin ensures minimal impact not only on analysis results, but also on analyzer electrodes, extending their service life.
The use of citrate or EDTA as an anticoagulant is not recommended as they are acidic and may artificially lower the pH of the sample.
Gas exchange with the surrounding air must be excluded in order to avoid changes in the gas composition and distortion of the research results. The entry of an air bubble into a sample can cause a significant change in its gas composition: depending on the size and time of storage of the sample before analysis, the results of determining O2 and CO2 can be distorted by 10–25%.
Hemolysis in a sample can greatly distort the results of the analysis of some basic acid parameters (K+, Ca++, pCO2, BE, CO-Hb). For example, if only 1% of red blood cells are hemolyzed, the plasma potassium level will increase by 0.7 mmol/L (at Hct = 45%) due to the high concentration of potassium inside the cells. Despite the fact that the normal level of potassium in the blood of, for example, newborns is 3.7 - 5.9 mmol/l. Partially hemolyzed samples pose a particular danger of diagnostic error, since it is very difficult to detect slight hemolysis in whole blood.
The main cause of hemolysis is violations of the blood collection procedure: puncture of the vessel through, damage to surrounding tissues, incomplete evaporation of alcohol on the skin, excessively active aspiration, etc. Prolonged cooling of the blood sample (in an ice bath) also leads to hemolysis. The use of special systems for arterial blood, careful adherence to the rules for its collection and minimizing the sample storage time before analysis (if possible without refrigeration) helps to reduce the influence of these factors and prevent hemolysis.
Arterial blood collection systems must provide high sample stability. In this case, the serious condition of the patient should be taken into account and gentle methods should be used that will not cause him additional suffering.
1) Ordinary syringes, pre-rinsed with heparin solution.
A heparin solution (1) is drawn into a glass syringe using a separate needle. In this case, the inner walls of the syringe are moistened with heparin when the piston is pulled back. The needle is then replaced and the heparin solution is released upward (2). This procedure requires additional time at a time when the result is needed within 5-15 minutes.
Liquid heparin (Na heparin) mixes easily with blood, but errors may occur due to non-standard washing procedures and different “residual” doses of heparin in the washed syringe: unpredictable sample dilution and scattered results, changes in the levels of sodium and calcium ions. During such sample preparation, the parameters of the blood taken may change significantly. There is also a risk of hemolysis in the sample, which is typical for the syringe/needle system.
2) Special syringes, heparinized at the factory.
Calcium-balanced lithium heparin (Li heparin+) is applied to the inner walls of the syringe using dry spraying. This eliminates dilution errors associated with liquid heparin, maintains the stability of gases, pH, Na+, K+, Ca++, Hb, Hct and glucose, and also minimizes the number of manual manipulations and reduces the time to obtain results.
Arterial blood collection syringes have a volume of 1, 2 or 3 ml and must be filled to a certain level (more than half the volume) to achieve the optimal concentration of anticoagulant in the sample. After collecting the sample, you need to thoroughly mix the blood with the anticoagulant: invert the syringe 5 times and gently rotate the syringe between your palms for 5 seconds.
Filling syringes with arterial blood
Self-filling (gravity): the syringe piston is pre-set to the required volume, and after inserting the needle into the artery, the syringe is filled spontaneously - under blood pressure;
! contact of blood with air when filling a syringe
Aspiration: after puncture of the artery, the syringe plunger is slowly pulled back to the desired mark, and the syringe is filled with blood.
! piston movement - increased risk of hemolysis
3) Capillary - a device for collecting capillary blood.
The inside of the capillaries is evenly coated with balanced heparin; the sample is not subjected to the force of a piston during aspiration or the intense pressure of blood during self-priming. However, it should be remembered that capillary blood does not provide reliable information about acid-base balance at a given time. Therefore, capillaries are used to analyze acid-base balance only if it is difficult to take blood from an artery.
Vacuum blood collection systems cannot be used for acid base analysis because negative pressure changes pO2 and pCO2.
Thus, the best sample for acid base analysis - arterial blood - can only be obtained using a syringe. But a number of negative features of the syringe/needle system reduce the stability of the sample and the accuracy of the acid base analysis.
4) Arterial sampler –
a device for collecting arterial blood into a capillary.
It combines all the positive qualities of heparinized arterial syringes and capillaries, but without the classic problems of using a syringe/needle system. Essentially, it is a glass capillary placed in a protective plastic housing, to which a needle is attached to puncture the artery and draw arterial blood.
A fast, one-step, automatic blood filling procedure with minimal blood pressure ensures sample stability to the most stringent standards. The arterial sampler minimizes the patient's pain and can be used for acid base analysis in intensive care units, when examining elderly patients and newborns.
An arterial sampler is an ideal device for collecting arterial blood.
In the market of medical laboratory equipment, arterial samplers are represented (USA).
Arterial sampler OPTI ComfortSampler
Increased sample stability
• The curved channel of the capillary slows down the movement of blood, the capillary is filled with minimal blood pressure; minimized risk of hemolysis in the sample
• Contact of the sample with air is minimized, the gas composition of the blood in the sample does not change, the results of determining O2 and CO2 are not distorted
• Dry sprayed lithium heparin, balanced with calcium, as an anticoagulant
• Analysis errors associated with sample dilution with liquid heparin are eliminated; accurate determination of electrolytes (ionized calcium, etc.)
• Blood sample volume – no more than 240 µl
• When blood enters the capillary, it is isolated from the heat transfer of the body and instantly cools (small volume), metabolic processes in the sample slow down
• No ice cooling required if sample is examined within 30 minutes of collection
Exceptional Security
• The glass capillary is placed in a protective plastic case, which eliminates the possibility of injury from broken glass
Visibility
• Transparent design makes it easy to determine the moment of puncture of the artery and control the filling level of the capillary
Compatibility
• The arterial sampler can be used with any blood gas analyzer that is capillary compatible
• Thin needles with a short bevel are used with an arterial sampler, this reduces pain and the appearance of hematomas at the puncture site, which is the most important criterion for the patient
The arterial sampler can be recommended for use in intensive care units, as well as when examining elderly patients and children.
The OPTI ComfortSampler arterial sampler is registered by RU No. FZS 2010/06428, with an unlimited validity period. Among the arterial blood collection devices presented on the Russian market, there are no analogues from foreign or domestic manufacturers.
Arterial sampler OPTI ComfortSampler
provides excellent sample stability,
minimizes potential errors in acid base analysis,
ideal for examining critically ill patients,
elderly patients for children.
Literature
1. Procedures for the collection of arterial blood specimens; Approved Standard – Fourth Edition. NCCLS - CLSI Document H11-A4, Vol.24, No. 28 (2004);
2. Ensuring the quality of collection of primary biological samples for laboratory research in the provision of emergency and urgent care. Guidelines. – Moscow, 2021. – 26 p.
3. Approved IFCC recommendations on whole blood sampling, transport and storage for simultaneous determination of pH, blood gases and electrolytes / Burnett RW, Covington AK, Fogh-Andersen N, et al. Eur J Clin Chem Clin Biochem. 1995 Apr; 33(4):247-53.
4. Samples: from patient to laboratory / Guder V.G., Narayanan S., Visser G., Tsavta B. /trans. from English V.V. Menshikova. GIT VERLAG 2001, Russian Version by Becton Dickinson & Co. (2003). -105 s.
5. Gorn M.M., Heitz W.I., Swearingen P.L. Water-electrolyte and acid-base balance. Per. from English -M.-SPb.: “Publishing house BINOM” - “Nevsky Dialect”, 1999. -320 p.
6. Modern technologies of the preanalytical stage of studying blood gases and electrolytes. A. Zh. Gilmanov, Doctor of Medical Sciences, Prof. bash
Return
Indications for analysis
The first and most important reason is the diagnosis of respiratory failure, which allows us to identify disorders of the acid-base state of the blood, such as acidification - acidosis or alkalization, or alkalosis. Monitoring of blood gas composition is necessary for various serious diseases, for example, severe obstructive pulmonary lesions, idiopathic fibrosing alveolitis, and chronic renal failure. A blood gas test is also required for:
- monitoring patients over time with carbon monoxide poisoning;
- monitoring for the development of methemoglobinemia;
- in persons with low saturation.
This analysis is taken from patients on mechanical ventilation in intensive care units, and before surgery, this study is necessary to assess the risk of surgical intervention during thoracic operations on the lungs and chest organs, including the heart.
Fencing technique: features and differences
Unlike routine biochemical studies, blood gas analysis is performed not from a vein, but from an artery. This is a little more complicated than using venous access. The veins lie superficially, the movement of blood in them occurs under less pressure, and puncture of the arteries should only be carried out by a specialist. When preparing for an arterial puncture, it is imperative to prepare a heparinized syringe to prevent the blood from clotting. The analysis must be taken from the brachial, radial or femoral artery.
Most often, a sample is taken from the radial artery on the nondominant arm. Thus, from an ordinary right-handed adult, blood is taken from the radial vein of the left arm. Since the arteries lie much deeper, taking a sample of such blood can be much more painful than during puncture of a vein. Local anesthesia is necessary, for example, the introduction of 1 ml of a one percent solution of lidocaine. A sign of correct entry into the artery is the penetration of scarlet, pulsating blood into the syringe under pressure.
Blood sampling for gases should be carried out by an experienced specialist to avoid the formation of air bubbles and to avoid contact of the arterial blood sample with ambient air.
After the sample is taken, the blood is carefully mixed so that the red blood cells do not settle, and is always mixed with heparin. Usually the syringe is already flushed with heparin solution. It must be remembered that heparin itself has a pH level close to 7, and if you take more of it than necessary, you may get a false blood pH result. Therefore, recently special syringes have been produced for collecting arterial blood for gases that contain dry heparin, which is neutral in relation to blood serum pH.
It is very important that the blood should be delivered to the analyzer as quickly as possible after collection, within 15 minutes. If long-term transportation is necessary, the sample must be cooled. Indeed, if room temperature is maintained, red blood cells will consume oxygen in the sample, which will lead to a decrease in its partial pressure. In addition, lactic acid will be formed, which can acidify the sample, thereby simulating the occurrence of metabolic acidosis.
How is the procedure done?
There is no special preparation for the procedure. Patients are not given any restrictions on drinking or eating before the test. The oxygen concentration should remain constant for 20 minutes before analysis; if the test is to be performed without oxygen saturation, the gas should be turned off for 20 minutes before the test is performed. The patient should breathe normally during the test. A blood sample is obtained by arterial puncture (usually in the wrist, although it can be done in the groin or arm). If a puncture is required, the skin over the artery is cleaned with an antiseptic. The healthcare professional then collects the blood using a small, sterile needle attached to a disposable syringe. The patient may feel a short pulsation or cramp at the puncture site. Once the material has been collected, it should be transported to the laboratory for analysis as soon as possible.
After the blood has been drawn, the doctor or patient presses cotton wool against the puncture site for 10-15 minutes to stop the bleeding, and then wraps it tightly with a bandage. The patient should rest quietly after completing the procedure. Health care providers will watch for signs of bleeding or circulation problems. The risks of getting them when the test is performed correctly are very low. Includes bleeding or bruising at the site of blood donation or after some time. Very rarely there may be a problem with circulation in the puncture area.
Normal blood gases in adults and children
The normal range of blood gases lies in the following values:
- the partial pressure of oxygen should be more than 80 mmHg in arterial blood when breathing normal atmospheric air;
- the partial pressure of carbon dioxide should be in the range from 35 to 45 mmHg in the same arterial blood, or 4.7 6 kilopascals;
- pH, which is a dimensionless quantity (negative logarithm of proton concentration), lies within narrow limits - from 7.35 to 7.45;
- The bicarbonate concentration is expressed in molar units and averages from 22 to 28 millimoles per liter.
The following are the corresponding values for children:
- pH 7.31-7.47;
- partial pressure of CO2 3.8-6.5 kPa (28-49 mmHg);
- partial pressure of O2 - 4.3-8.1 kPa (32-61 mmHg);
- bicarbonate - 15-25 mmol/l
Hypoxemia, hypercapnia and their causes
The most common cause of tissue hypoxia and disturbances in blood gas analysis is hypoxemia, which occurs with various pathologies of the lung tissue. First of all, this is chronic obstructive pulmonary disease, as well as the proliferation of pulmonary connective tissue to the detriment of the alveolar tissue. This is the so-called pulmonary fibrosis or cirrhosis of the lung, or a pathology such as interstitial lung disease (idiopathic fibrosing alveolitis).
In some cases, the cause of hypoxemia and a decrease in the partial pressure of oxygen in arterial blood is a pronounced shunt, when blood from the arterial and venous systems mixes. Most often this occurs with a massive defect of either the interatrial or interventricular septum. And this condition is normal in children; the oval window gradually heals, and the arterial and venous blood flow are completely isolated from each other. However, it is known that in 25% of adults this window does not close, but the discharge of blood is so insignificant that it has no hemodynamic and clinical signs.
A right-to-left shunt may also occur with other congenital diseases of the heart and large vessels, for example, with such a rather severe pathology as respiratory distress syndrome.
Hypoxemia can occur not only due to hypoventilation and shunting, but also due to ventilation-perfusion disorders of a different nature, of which the most common are bronchial asthma, pneumonia, and sarcoidosis. Finally, this type of disorder also includes all types of anemia, in which there is not enough hemoglobin per unit volume of blood, and secondary hypoxemia develops.

A characteristic clinical sign of tissue hypoxia of all forms is cyanosis, or cyanosis. Most often, diffuse cyanosis is clearly visible in the area of the nail plates, earlobes, and in the area of the nasolabial triangle. It becomes noticeable at a partial pressure of oxygen equal to 50 mmHg, and a distinct cyanosis, which is striking to a non-specialist, occurs with deep hypoxemia, in which the partial pressure of oxygen is less than 40 mmHg.
Blood gas studies and pulse oximetry
Pulse oximetry and blood gas testing procedure
Among the most accurate methods for determining how well the body is supplied with oxygen, a special place is occupied by the study of blood gases and pulse oximetry.
Basic task
Blood gas testing is used for diseases of the cardiovascular and respiratory systems. This is done to determine the oxygen saturation of the body. Carrying out the research is quite simple. To do this, a puncture method is used, in which blood is taken directly from the artery. Due to this, the method belongs to the category of invasive. After the artery is punctured, a bandage should be applied to it for about 10-15 minutes. This is necessary to prevent bleeding. The sample obtained during the sampling process is immediately sent to the laboratory for testing. At this stage, the amount of gas in the blood is determined. Here, the determination of carbon dioxide, partial pressure, pH indicators, the quantitative composition of bicarbonates and how saturated hemoglobin in red blood cells necessarily occurs. The following are considered normal values:
- PaCO2 - 34-45 mm Hg;
- PaO2 - 75-100 mm Hg;
- SaO2 - 94-100%;
- pH of 7.35-7.45;
- HCO3 - 22-26 mEq/liter.
Pulse oximetry method and its information content
The most accurate method to recognize respiratory failure and assess the general condition of the respiratory system is to study blood gases. As soon as a person begins to experience respiratory failure, the process of developing hypoxia (decreased oxygen levels) and hypercapnia immediately occurs - an increase in the amount of carbon dioxide in the composition.
The presented research method is used to recognize restrictive and obstructive pulmonary diseases in a chronic form. These include diseases such as sarcoidosis, bronchial asthma, tuberculosis, and occupational lung diseases. The research procedure takes place only on the hospital premises.
To conduct the study, the patient does not require special preparation. If a person uses anticoagulants, anti-inflammatory drugs and aspirin, you need to warn the doctor about this. As for the dangers of this procedure, this includes the possibility of bleeding after the puncture.
Features of pulse oximetry
Pulse oximetry is a method that can be used to determine the oxygen saturation of hemoglobin in the blood. For this, a special device called a pulse oximeter is used. Depending on the amount of oxygen, the change in blood color allows you to determine the necessary parameters. The convenience of this method is that there is no need to collect venous blood.
Conducting the research and its informativeness
During the procedure, a special sensor is placed on the patient’s finger, in which the main source is light. Passing through the phalanx and capillaries, the process of recording the change in blood color occurs depending on how saturated it is with oxygen. The data is recorded on the device screen in the form of saturation curves. To get the most accurate result, it is necessary to ensure complete immobility of the finger. The normal rate should be 95-98%. To recognize respiratory failure and other problems with the respiratory system, the blood saturation method is informative. If the quantity is insufficient, the indicator drops below 95%. This method is often used by anesthesiologists during surgical interventions. No special preparation is needed for the procedure. The method does not lead to complications and is safe for the human body.
Pulse oximetry: principle of operation
Pulse oximetry is an extremely accessible method of patient monitoring. This is especially important when there is limited funding for a medical institution. Allows you to monitor several parameters of the patient’s condition at once. Initially, the use of the most accurate pulse oximeters was required in intensive care units, then everywhere. But proper use of pulse oximetry requires special skills. If used incorrectly in a general therapy department, there may be a threat to the life and health of the patient. Let's consider the operating principle of a pulse oximeter, the features of the modern method, and possible limitations. And also, what alternatives to this method exist.
Principle of operation
A pulse oximeter is a high-precision device that measures the degree of oxygen saturation of arterial hemoglobin. The technology is based on 2 principles: absorption of light by hemoglobin and pulsation of the light signal as it passes through tissue, which occurs due to changes in the arterial bed. This component can be separated from the non-pulsating component using a special microprocessor. When used correctly, oximetry becomes the most useful method for monitoring the state of the cardiorespiratory system. As a result, 2 indicators are displayed on the monitor:
- hemoglobin oxygen saturation of arterial blood;
- heart rate (measured over 5 - 20 seconds).
Several factors influence the correct operation of the device. These include external light, pulse frequency and rhythm, hand tremors, and pathological hemoglobin. Reliability can also be affected by vasoconstriction, pathological hemoglobin, and characteristics of the heart.
The pulse oximeter only shows the level of ventilation of the blood, but not the level of ventilation. With low qualifications of the medical worker, this often creates a false picture when inhaling oxygen. In such a situation, there is a risk of missing the initial symptoms of hypoxia, which occurs with airway obstruction.
What does a pulse oximeter measure?
A pulse oximeter consists of several elements:
- sensor for collecting indicators (attached to a finger, earlobe or nose);
- microprocessor for processing results;
- display for processing the results.
The device shows the average amount of oxygen that is associated with each hemoglobin molecule. Data is displayed on the monitor simultaneously with an audio signal. Its height varies depending on the level of saturation. Pulse rate is measured based on the number of beats per minute. The pulse oximeter does not provide information on the following indicators:
- blood oxygen level;
- amount of dissolved oxygen;
- tidal volume;
- breathing rate;
- cardiac output value;
- arterial pressure.
Systolic pressure is determined by the appearance of a wave on plethysmography during cuff deflation.
Principles of modern pulse oximetry
The principle of modern pulse oximetry is the relationship between partial pressure of oxygen and saturation. This indicator is reflected in the hemoglobin dissociation curve. Under different conditions, it can move to the right or left. For example, this can occur during blood transfusion.
Operating principle of a pulse oximeter:
Oxygen is transported through the bloodstream mainly in the form of hemoglobin. One molecule of hemoglobin can carry 4 molecules of oxygen and in this case it will be 100% saturated. The average percentage of saturation of the population of hemoglobin molecules in a certain volume of blood is the oxygen saturation of the blood.
The sensor contains two LEDs, one of which emits visible light in the red spectrum (660 nm), the other in the infrared spectrum (940 nm). The light passes through the tissue to the photodetector, and part of the radiation is absorbed by the blood and soft tissues, depending on the concentration of hemoglobin in them. The amount of absorbed light of each wavelength depends on the degree of oxygenation of hemoglobin in the tissues.
- The microprocessor is able to isolate the pulse component of blood from the absorption spectrum, i.e. separate the arterial blood component from the permanent venous or capillary blood component. The latest generation microprocessors are able to reduce the effect of light scattering on the operation of the pulse oximeter.
Multiple divisions of the signal over time are accomplished by cycling the LEDs: red turns on, then infrared, then both turn off, and so on many times per second. This eliminates random background noise.
A new feature of microprocessors was quadratic multiple division. The red and infrared signals are phase-separated and then recombined. With this option, interference from motion or electromagnetic radiation can be eliminated, since they cannot occur in the same phase of the two LED signals.
Like heart rate, saturation is calculated on average in 5-20 seconds. The first indicator is calculated by the number of LED cycles and strong pulsating signals over a certain period of time. Based on the proportion of absorbed light of each frequency, the microprocessor calculates their coefficient. The pulse oximeter memory contains a series of oxygen saturation values obtained in experiments on volunteers with a hypoxic gas mixture. The microprocessor compares the resulting absorption coefficient of the two wavelengths of light with the values stored in memory. It is unethical to reduce oxygen saturation in volunteers below 70% in clinical studies. Because of this, a saturation value below 70% obtained from a pulse oximeter is not reliable. Reflected pulse oximetry uses this type of light. Can be used proximally, for example on the forearm or anterior abdominal wall. The operating principle is the same as that of a transmission pulse oximeter. A significant drawback is the difficulty of attaching to the body.
Causes of hypercapnia
But gas exchange can also be impaired due to high partial pressure of carbon dioxide in the blood, which is called hypercapnia. Most often, hypercapnia is toxic or medicinal in nature, or is associated with impaired airway patency. Quite often, the cause of long-term hypercapnia is insufficient pulmonary ventilation due to weakness of the respiratory muscles. It is caused by myasthenia gravis, muscular dystrophy, ascending Landry's paralysis, or Guillain-Barré syndrome, and some types of multiple sclerosis.
Such chronic hypercapnia can also be caused by scoliosis, various chest injuries, as well as a condition after lung surgery, when the patient simply physically has to breathe “shallow” in order to avoid suture dehiscence.
Mixed conditions often occur when “dead space” accumulates in the lungs, through which practically no air flow moves. The already mentioned pulmonary cirrhosis and fibrosis, obstructive disease, bronchial asthma, cystic fibrosis and other pathologies lead to such processes. In some cases, the production of carbon dioxide also increases in conditions not related to the pathology of the bronchopulmonary system. This is fever, sepsis, convulsive syndrome or excess load of carbohydrate foods.
What can affect the result?
Blood gas analysis is a very specific and highly sensitive test. Therefore, necessary conditions must be observed to avoid false results. First of all, it is necessary to exclude fever and elevated temperature; arterial blood sampling should be carried out at normal body temperature.
In case if:
- in the patient it is above 37 degrees, then the partial pressure of both oxygen and carbon dioxide will be higher than necessary, and the acid-base balance will be shifted to the acidic side, that is, towards acidosis;
- If the patient has hypothermia, or cooling of the body, the reverse process will take place: the partial pressure of gases will fall, and the pH level will increase in the direction of alkalization of the blood.
Other factors also influence the test results. So, in some cases, it is necessary to adjust their accuracy taking into account the patient’s age, and there are special calibration tables. After all, the older the patient, the lower the partial pressure of oxygen in the blood. It is very important to calibrate the analyzer as often as possible with each sample taken.
As a result, all this leads to the fact that the cost of research is not cheap. Thus, the average price in private clinics ranges from 1,500 rubles to 3,000 rubles.
Of course, you can find a state clinic in which the price of the test will be 100 rubles, but you need to remember that this is about the same as treatment from a dentist at your own site under an insurance policy, when the patient pays only for cheap consumables.
Test results
The results of the analysis consist of several indicators that will help determine how efficiently the blood system functions. They also express the level of oxygen saturation in the body, which is very important for internal organs. The main criteria are:
Partial pressure (PP)
Partial pressure is a way of estimating the number of molecules of a particular gas in a mixture of gases. This is the amount of pressure of a particular gas compared to the total pressure. For example, we normally breathe air that at sea level has a pressure of 100 kPa, oxygen is 21% of 100 kPa, corresponding to a partial pressure of 21 kPa. In blood gas testing, Henry's law is used to determine the partial pressures of gases in the blood. This law states that when a gas dissolves in a liquid, the partial pressure (that is, the concentration of the gas) inside the liquid is the same as that of the gas in contact with the liquid. Therefore, the partial pressure of gases in the blood can be measured. You will see a column marked PaO2 - partial pressure of oxygen in arterial blood and PaCO2 - partial pressure of carbon dioxide.
Basic excess (BE)
This is the amount of strong base that must be added or subtracted from a substance to return the pH to normal (7.40). A value outside the normal range (-2 to +2) indicates a metabolic cause of acidosis or alkalosis.
Bicarbonate (HCO3)
Bicarbonate is produced by the kidneys and acts as a buffer to maintain pH. The normal range for bicarbonate is 22-26 mm/L. If there are additional acids in the blood, bicarbonate levels will drop as ions are used to buffer these acids. If there is chronic acidosis, the kidneys produce slightly more bicarbonate to keep the pH normal. It is for this reason that increased bicarbonate can be observed in chronic respiratory failure type 2, when the pH remains normal despite increased CO2.
Electrolytes
A venous or arterial gas test is a good way to quickly check your potassium and sodium levels. This is especially important in the direct treatment of cardiac arrhythmias, as it gives immediate results.
Lactate
Produced as a by-product of anaerobic respiration. Elevated lactate can be caused by any process that causes tissue to use anaerobic respiration. This is an effective indicator of poor tissue perfusion.
Glucose
Glucose is especially important when treating a patient who suffers from loss of consciousness or frequent seizures. This is also necessary for patients with suspected diabetes. Glucose may be elevated in patients with severe sepsis or other metabolic stress.
Other Analysis Components
They are rarely violated and often overlooked. However, it is important to notice if they are outside the norm. This is especially true in the case of carbon monoxide, as there may be other people at risk.
Carbon monoxide (CO)
Typically CO is <10%. In city dwellers or smokers, levels may rise up to 10%, but levels >10% indicate poisoning, usually due to poorly ventilated boilers or old heating systems. At levels of 10-20%, symptoms such as nausea, headache, vomiting and dizziness will be observed. At higher levels, patients may experience arrhythmia, cardiac ischemia, respiratory distress, and mild seizures.