The human body is inhabited by trillions of microorganisms, the totality of which is called the microbiome. The microbiome performs many important functions - from the synthesis of vitamins to the breakdown of complex food components. “Healthy” microflora constantly competes for limited nutritional resources with pathogenic microorganisms, thereby suppressing their growth. However, due to antibiotics or other reasons, the normal composition of the microbiome can be disrupted, allowing pathogens to multiply uncontrollably, causing disease. One such pathogen is the bacterium Clostridium difficile, the causative agent of pseudomembranous colitis. The fight against C. difficile is complicated by its resistance to most known antibiotics. But recently it was shown that the growth of C. difficile can be successfully suppressed not with drugs, but with the help of a related species - C. scindens. This discovery will serve as the basis for the creation of “smart” probiotic drugs: effective against C. difficile, but safe for beneficial microflora.
Figure 1. Correlation between the presence of specific bacterial taxa in the microbiome and resistance to C. difficile infection [3].
Antibiotics are effective in combating many deadly diseases, but at the same time they cause significant damage to the human microbiome. After a course of antibiotics, a person is usually more susceptible to infection by various pathogens. Clostridium difficile is a Gram-positive, motile bacterium, the main causative agent of acute nosocomial intestinal infections. Over the past 15 years, the number of deaths due to C. difficile has increased at least 10-fold, especially among elderly and frail people [1]. The fight against C. difficile is complicated by the fact that under unfavorable conditions this species forms spores that can withstand the action of antibiotics. This feature allows bacteria to re-colonize the intestines several weeks and even months after the end of treatment.
Recently, microbiome transplantation from healthy donors has been shown to cure severe C. difficile infections [2]. However, it remained unknown which members of the microbiome restore the body's resistance to C. difficile infection and by what mechanisms. And finally, an article has been published that sheds light on a rather unusual mechanism of interspecies interactions in the microbiome [3].
Of Mice and Men
C. difficile is not only a human scourge, but also a mouse one. To begin with, they tried poisoning the microbiomes of mice with different antibiotics and looked at how susceptibility to C. difficile changed from this. In general, antibiotics do not so much reduce the total number of bacteria in the intestines as significantly disrupt the balance of power - the relationship between different taxonomic groups. It turned out that susceptibility to C. difficile clearly correlates with an overall decrease in microbiome species diversity. It was possible to identify a handful of 11 conventional species (operational taxonomic units) associated with resistance to C. difficile infection (Fig. 1). Many of them also turned out to be clostridia (cluster Clostridium XIVa). The scientists focused on one taxon whose presence was most strongly correlated with resistance to C. difficile, even in animals with extremely low microbiome species diversity. The hero turned out to be Clostridium scindens.
Figure 2. Correlation between establishment of candidate species in the microbiome and resistance to C. difficile [3].
But that's in mice. How are things going for the person? To identify species associated with resistance to C. difficile infection, the microbiomes of patients undergoing allogeneic hematopoietic stem cell transplantation were examined. Most of them received chemotherapy and/or radiation therapy concurrently with a course of antibiotics at the time of transplantation. Weakened immunity and decreased microbiome diversity make these patients easy targets for C. difficile. In humans, we were able to find two main species with which C. difficile did not coexist. The most potent inhibitor was C. scindens, as in mice. As if it were a victory?
To test the inhibitory effect, C. scindens, along with several other promising bacteria, were introduced into the intestines of animals that had recently taken antibiotics (Fig. 2). It turned out that such microbial transplantation significantly alleviated the course of infection caused by C. difficile, and also had a positive effect on reducing mortality and increasing body weight compared to control. The most prominent resistance to C. difficile was, as expected, provided by C. scindens. The engraftment of transplanted bacteria in the microbiome was monitored by the presence of the corresponding 16S rRNA gene. Resistance to C. difficile increased in direct proportion to the abundance of C. scindens. That is, improving the establishment of C. scindens may increase protection against C. difficile. It is noteworthy that such a careful replacement of a bad clostridia with a very similar, but good one, does not disturb the existing balance in the microbiome (neither qualitative nor quantitative). A kind of antidote therapy, only at the microbiome level. All this opens up sparkling horizons for the development of safe drugs against C. difficile. But how exactly does C. scindens fight C. difficile?
Clostridia. Always there, always on the alert
Back to list Previous article Next article
11.07.2012
Tags:
bacteria, clostridia, microflora
( 7 ratings, average: 4.71 out of 5)
Botulism, tetanus, gas gangrene ... If not all, then very many people have heard about these diseases. But if there are vaccines against tetanus, for example DTP (adsorbed pertussis-diphtheria-tetanus serum), then there are no such vaccines against pseudomembranous colitis, necrotic enteritis, botulism or gas gangrene. And all these diseases are caused by bacteria of the genus Clostridium.
One of the most common types of clostridia is Clostridium difficile . It is sown from soil and water (including sea water). Due to the ability to form endospores (become covered with a shell and survive an unfavorable period in a state of a kind of “hibernation”), this species can survive for a long time, up to two months, in the external environment. Moreover, in the endospore state, Clostridium difficile can even withstand boiling. Clostridium difficile is naturally very sensitive to antibiotics , so treatment with antibiotic therapy often fails.
Clostridium difficile is part of the normal flora (a representative of the normal microbial composition) of the gastrointestinal tract, living mainly in the large intestine . Although some of its representatives are found in the mouth, and in the small intestine, and in the vagina of women. Most often, Clostridium difficile is found in the intestines of newborns (about half of infants), and of children over 2 years of age and adults, every 10 are the owner of this bacterium.
Clostridium difficile causes diseases such as pseudomembranous colitis and antibiotic-associated diarrhea. The pathogenic (pathogenic) properties of this clostridia are due to the release of toxins A and B, as well as a protein that inhibits intestinal motility (contractile function) .
Antibiotic dissociated diarrhea (abbreviated as AAD) can be caused not only by Clostridium difficile , but also by many other microorganisms (Salmonella, Candida, Clostridium perfringens, Staphylococcus aureus, Klebsiella). This type of diarrhea is the most common nosocomial infection and this trend is due to the fact that in hospitals, firstly, a large mass of sick and healthy people are concentrated. And secondly, many antibiotics and disinfectants are used here, which contribute to the formation of generations (generations) of microorganisms resistant to antibiotics. Therefore, about a million cases of AAD are registered annually in the world. The occurrence of antibiotic-associated diarrhea is due to the fact that antimicrobial drugs suppress both pathogenic and normal microflora. Even a single dose of broad-spectrum antibiotics can cause the development of AAD.
Clostridia
Despite this, infants practically do not suffer from antibiotic-associated diarrhea caused by Clostridium difficile . This is due to the fact that children receive enough immune factors from their mother’s milk that can inhibit the proliferation of Clostridium difficile. Moreover, the infant body does not have such a quantity of concomitant opportunistic microflora that weakens the immune system.
Antibiotic-dependent diarrhea can have different symptoms and course: from mild diarrhea to severe pseudomembranous enterocolitis (PME). The latter is most often caused by Clostridium difficile.
During the course of the disease, the following symptoms :
- frequent and profuse watery diarrhea , in which bloody, mucous and purulent inclusions are sometimes observed;
- fever with high temperature (up to 40°C);
- abdominal pain , which can be either constant or “cramping.”
If left untreated, the death rate is up to 30%.
A characteristic feature of diseases caused by Clostridium difficile is that about a quarter of cases recur over time ( disease relapse ). The cause of this phenomenon is clostridia spores that survive the treatment period or re-infection. As a rule, after treatment, patients recover or feel much better, but after a few days (from 3 to 7) a relapse develops.
Another clostridia that can cause gastrointestinal diseases such as foodborne illnesses and necrotic enteritis is Clostridium perfringens .
Necrotic enteritis is characterized by the formation of ulcers and erosions and destructive (destructive) changes in the mucous membrane. The first symptom of the disease is the appearance of areas of hemorrhagic necrosis in the initial parts of the jejunum . These areas are red . There is also a narrowing of the intestinal lumen at the site of inflammation , due to swelling of the wall. (blockage) of small blood vessels (arterioles) occurs The patient has chills and fever, vomiting and bloody foamy diarrhea .
Clostridium perfringens produces enzymes that break down proteins (proteinase), collagen (collagenase), and hyaluronic acid (hyaluronidase).
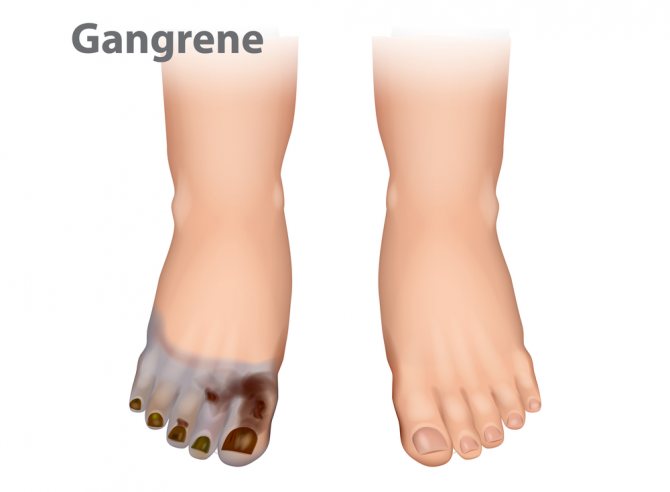
Gangrene, caused by Clostridia
They synthesize proteinases, lecithinase, collagenase, hyaluronidase and other aggressive enzymes . Clostridium perfringens also produces toxins, including those that dissolve red blood cells (hemolytic), in particular, β-toxin, as well as proteins that can form holes in the membranes of intestinal cells and thus cause cell dehydration and the release of potassium ions.
Prevention of diseases caused by clostridia primarily consists of observing sanitary and hygienic standards and rules: washing and scalding vegetables and fruits; long-term heat treatment. But it is also important to normalize the microflora and strengthen the immune system. Moreover, both in the process of treatment and in the process of preventing these diseases. Limited and strictly supervised use of antibiotics by doctors and avoidance of self-medication are also very important for the prevention of ADD and other diseases associated with clostridia.
Back to list Previous article Next article
Mechanism of inhibitory action
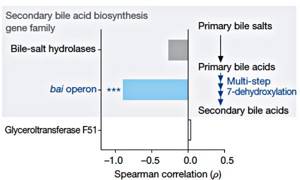
Figure 3. Correlation between resistance to C. difficile and the presence of gene families required for the synthesis of secondary bile acids [3].
It is known that some secondary bile acids can impair the growth of C. difficile in vitro [4]. A very large number of microbiome bacteria have the bsh gene, which encodes bile acid hydrolase. However, a rare bacterium (an abysmally small fraction of microbiome organisms) has all the genes needed to carry out the complete bile acid biosynthetic pathway. A rare bacterium, of course, turned out to be C. scindens, which has a 7α-hydroxysteroid dehydrogenase gene critical for the synthesis of secondary bile acids (Fig. 3). It is on this unique biochemical feature that the protective mechanism of C. scindens against C. difficile is based. The introduction of C. scindens into the microbiome of animals susceptible to C. difficile restores the required level and ratio of secondary bile acids: deoxycholic acid (DCA) and lithocholic acid (LCA). Both of these acids inhibit the growth of C. difficile in proportion to their concentration. Notably, C. scindens increases secondary bile acids to physiological levels and inhibits the growth of C. difficile even in animals treated with antibiotics. This mechanism is conservative - it is implemented both in the mouse microbiome and in the human microbiome.
In what cases is research usually prescribed?
Clostridia toxin testing is prescribed for the following conditions:
- diarrhea in patients taking or undergoing a course of antibiotics (especially after clindamycin, lincomycin, synthetic penicillins, cephalosporins), as well as diarrhea in those admitted to the hospital 72 hours after hospitalization;
- suspicion of pseudomembranous colitis in patients at high risk: over 65 years of age, long-term hospitalization, surgical operations, the presence of malignant tumors, renal failure, inflammatory diseases of the colon, chemotherapy, hypoalbuminemia.
Therapeutic perspectives
C. scindens inhibits the growth of C. difficile through the synthesis of secondary bile acids*. However, the use of these acids as an independent medicine is unsafe, since some of them are associated with the development of duodenal cancer [5]. It is much more effective to use C. scindens itself and work, for example, on improving the survival rate of this species in the microbiome. Other bacteria may help here through additional mechanisms: competition for carbohydrates with C. difficile, activation of the host immune defense, or production of antimicrobial peptides. This rational approach will allow C. scindens to be used as an effective weapon against C. difficile without harming the rest of the microbiome.
* - About the dirty tricks of intestinal saboteur microbes that reduce the pool of bile acids and stimulate the development of atherosclerosis, read the article “Do not trust advertising, or the potential connection between the metabolism of L-carnitine and the development of atherosclerosis” [6] - Ed.
Literature
- Lucado J., Gould C., Elixhauser A. (2012). Clostridium difficile infections (CDI) in hospital stays. Statistical brief No. 124 (Healthcare cost and utilization project of the Agency for Healthcare Research and Quality (AHRQ), USA);
- de Vrieze J. (2013). Medical research. The promise of poop. Science. 341, 954–957;;
- Buffie CG, Bucci V., Stein RR, McKenney PT, Ling L., Gobourne A. et al. (2015). Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature. 517, 205–208;;
- Sorg JA, Sonenshein AL (2008). Bile salts and glycine as cogerminants for Clostridium difficile spores. J. Bacteriol. 190, 2505–2512;
- Bernstein H., Bernstein C., Payne C. M., Dvorakova K., Garewal H. (2005). Bile acids as carcinogens in human gastrointestinal cancers. Mutat. Res. 589, 47–65;;
- Do not trust advertising, or Potential connection between L-carnitine metabolism and the development of atherosclerosis.