Complexes with this research
Pregnancy planning.
Clinical indicators RUR 6,630 Composition Causes of hair loss RUR 2,500 Composition
Biochemistry of blood. 19 indicators Advanced biochemical blood test 6,280 R Composition
IN OTHER COMPLEXES
- Examination during pregnancy. 3rd trimester 9,620 RUR
- Men's check-up No. 1 RUB 18,570
- Women's check-up No. 1 RUB 19,290
- Biochemistry of blood. 8 indicators 990 R
- Examination during pregnancy. 1st trimester 16,690 RUR
Detailed description of the study
Serum iron is a microelement that is not produced in the human body independently, but comes only from the outside with food.
Iron is included in:
- Hemoglobin, which is the main carrier of oxygen from the lungs to tissues and organs;
- Myoglobin, which is located in the muscles and is responsible for creating an oxygen reserve.
Iron also participates in cellular metabolism, in the functioning of the immune system, is part of some enzymes, ensuring the normal occurrence of metabolic reactions in them, and also participates in DNA synthesis.
In total, the body contains no more than 5 g of iron. It comes from food from animal and plant sources. This is the so-called heme (found in meat products, as part of hemoglobin) and non-heme (enzymes and proteins of plant foods) iron. Of the total amount of iron that enters the body, only about 10% is absorbed. The absorption process occurs in the duodenum.
In human blood plasma, most of the iron is in the oxidized trivalent state, bound to the protein transferrin. Transferrin transports iron to tissues and organs. Most of the incoming iron goes into the formation of hemoglobin, and the rest is deposited (stored). The main depot of iron in the body is the special protein complexes “ferritin” and “hemosiderin”. From here, iron is consumed if it decreases in the blood serum. The restoration of iron in the blood serum depends on the degree of depot filling. With prolonged iron deficiency, iron reserves are depleted, which can lead to anemia. This regulatory mechanism ensures self-preservation.
Serum iron concentration is an important biochemical indicator, changes in which may indicate possible problems in the metabolism of this microelement.
Iron deficiency is more common than iron excess. As a rule, a person does not notice that he is developing anemia - a pathological decrease in the concentration of hemoglobin in the blood. This usually becomes noticeable when the hemoglobin concentration drops below 100 g/l. Then the person may complain of increased fatigue, dizziness, hair loss, pale skin and brittle nails. In some cases, people report a tingling and burning sensation on the tip of the tongue.
If you do not take any action aimed at restoring the iron depot, shortness of breath, absent-mindedness, and rapid heartbeat (tachycardia) further develop.
Similar conditions are observed in cancer (especially if the intestines are affected), iron deficiency anemia, jaundice, acute and chronic infections, peptic ulcers and ulcerative colitis. Iron deficiency can often be caused by chronic blood loss from hemorrhoids. In case of celiac disease, Crohn's disease and in the postoperative period, anemia is also diagnosed. Iron concentrations in women vary depending on the phase of the menstrual cycle.
There is also the so-called “anemia of pregnancy” - a condition in which the concentration of serum iron in pregnant women decreases. As a rule, in the second trimester, when the fetal iron depot is formed. This condition also requires special monitoring.
Iron overload is less common and is characterized by the following symptoms: abdominal and joint pain, heart rhythm disturbances, and fatigue, similar to anemia.
For the normal functioning of all organs and systems, it is necessary that at least 1 mg of iron be supplied with food per day. Foods rich in this microelement: meat and offal (pork liver), legumes (lentils, beans), greens (spinach, celery), fruits (pomegranate, apples). Ascorbic acid improves the absorption of iron, so it is recommended to use it together with iron-containing foods. Dairy products and caffeine can interfere with iron absorption.
Indications for the study
In the early stages of microelement deficiency, clinical manifestations are usually absent. As the situation worsens, the following health complaints appear:
- dyspnea;
- weakness, constant feeling of fatigue;
- pale skin;
- pain in the chest area;
- attacks of dizziness;
- frequent headaches.
Sometimes, with a lack of a microelement, such characteristic symptoms as a burning sensation on the tongue, the appearance of unusual food cravings (for example, there is a desire to eat clay), and small ulcers in the corners of the mouth are observed. Often, a blood test for iron is prescribed after detecting abnormalities in the results of the blood test, which is performed during a preventive examination or for other reasons.
References
- Federal clinical guidelines for the diagnosis and treatment of iron deficiency anemia, 2015. - 58 p.
- Klochkova-Abelyants, S.A., Surzhikova, G.S. Iron deficiency anemia and anemia of chronic diseases: some aspects of pathogenesis and prospects for differential diagnosis. — Medicine in Kuzbass, 2021. — No. 3. — P.25-28.
- Tiglis, M., Neagu, T., Niculae, A. et al. Incidence of Iron Deficiency and the Role of Intravenous Iron Use in the Perioperative Period, 2021. - Vol. 56(10). - P. 528.
Iron deficiency in pregnant women
Iron deficiency anemia is one of those rare complications of pregnancy that can be easily prevented during the planning stage. Even healthy pregnant women are at risk of developing anemia due to physiological hemodilution and the depletion of iron stores for fetal growth and maternal erythropoiesis [1]. Considering the fact that the developing fetus is very demanding on the concentration of oxygen and iron in the blood supplied to it, the placenta is able to increase the number of receptors for iron and store it in resident reticuloendothelial cells [1,3]. A distinction should be made between iron deficiency and iron deficiency anemia (IDA). The distribution of iron in the body is predominantly in favor of heme iron, therefore, the development of anemia against the background of iron deficiency is a signal of a pronounced deficiency of the microelement in the depot [3]. In this regard, three stages of deficiency are distinguished: pre-latent - a decrease in reserve iron without reducing its expenditure on erythropoiesis, latent - depletion of depots and deficiency of transport iron, without signs of anemia and manifest or IDA [8].
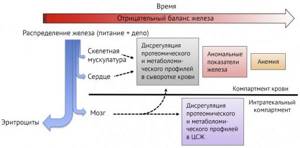
Figure 1 | Changes in the body at various stages of iron deficiency. The blue arrow indicates the distribution of iron entering the body - primarily to erythropoiesis, then to myoglobin synthesis and enzyme systems. The red arrow indicates the progression of the deficiency over time [3].
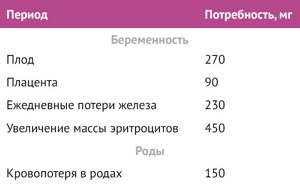
The need for iron varies at different stages of pregnancy. In the first trimester, the daily requirement is reduced compared to the non-pregnant state, mainly due to the absence of menstrual blood loss, as well as due to a decrease in erythropoiesis. In the second trimester, circulating blood volume increases by 45%, plasma volume by 50%, and red blood cell mass by 35%, therefore, the need for iron increases. In the third trimester, the fetus's need for iron increases to its maximum, and blood loss during childbirth takes away about 150 mg of iron [4].
Table 1 | Average increase in iron requirements during pregnancy and the postpartum period for women with a pre-pregnancy body weight of 55 to 60 kg [4,9]
Risk factors for the development of iron deficiency and iron deficiency anemia in women of childbearing age [4,8]:
- diet disorders (vegetarianism or veganism, insufficient consumption of red meat, anorexia, bulimia, excessive consumption of calcium, tea, coffee);
- nutrient malabsorption disorders (Crohn's disease, celiac disease);
- parasitosis (hookworm, giardiasis, cestodosis, schistosomiasis);
- chronic diseases (malaria, HIV, hemoglobinopathies, autoimmune diseases, cancer);
- complications of pregnancy (vomiting of pregnancy, preeclampsia, cholestatic hepatosis);
- short intervals between pregnancies (less than a year);
- menorrhagia;
- blood donation.
Manifestations of deficiency are extremely nonspecific and can be attributed by a woman to the pregnancy itself: fatigue, weakness, irritability, poor concentration, pale skin and mucous membranes, headache, dizziness, tachycardia, shortness of breath, tired legs. Some women note a predilection for specific foods: raw meat, raw cereals, chalk, soil [1,8,10].
There are certain dangers associated with iron deficiency: an increased risk of postpartum depression, postpartum hemorrhage (presumably due to a decrease in uterine contractility in conditions of oxygen deficiency in the tissues), some studies also indicate the risk of developing postpartum sepsis [1,10]. Women with anemia are more likely to undergo blood transfusions and deliver by caesarean section [2]. Predominantly in low-income countries, hemoglobin levels <70 g/L are associated with a twofold increase in maternal mortality [1,10]. In the postpartum period, anemia is associated with increased fatigue, depressive disorders, decreased cognitive abilities, impaired lactation, and early cessation of breastfeeding [10].
Iron in the fetal body is divided into heme iron, non-heme iron and storage, located mainly in the liver. Red blood cells contain 55 mg/kg of iron in the form of hemoglobin, the normal concentration of which in full-term infants is 133–184 g/l. The depot stores about 12 mg/kg of iron in the form of ferritin, the normal concentration of which in the umbilical cord blood of a full-term baby is 170 mg/l. The smallest amount of iron (about 8 mg/kg) is involved in metabolic processes and oxygen transport in muscles. Non-heme iron deficiency is difficult to track due to the lack of specific biomarkers; in the laboratory it is detected only in conjunction with iron deficiency in the depot and, as a consequence, reduced ferritin. Ferritin concentrations <76 mg/L are associated with impaired neurological function in the newborn [3]. In cases of mild to moderate deficiency in the mother, compensatory mechanisms maintain adequate iron supply to the fetus, but severe deficiency, leading to the development of IDA in the mother, leads to the development of iron deficiency in the child [5].
Regulation of iron bioavailability during pregnancy depends in part on maternal hepcidin concentrations. Hepcidin is a hormone regulator produced in the liver and regulating the concentration of iron in plasma and its distribution in tissues. Hepcidin acts by inhibiting the entry of iron into the blood, affecting absorption in the intestine, the release of iron from macrophages and its mobilization from the depot. The hormone acts through the ferroportin (Fpn) receptor, which is expressed in all tissues that export iron into the plasma. The binding of hepcidin and ferroportin triggers the sequestration of iron in target cells and a decrease in its entry into the plasma; therefore, there is an inverse relationship between the concentration of hepcidin and sufficient iron uptake by tissues, including bone marrow and placenta [9].
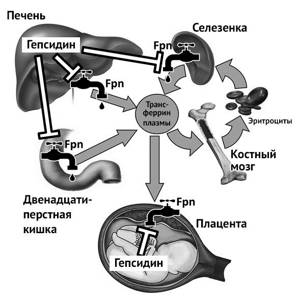
Figure 2 | Regulation of iron homeostasis in the body of a pregnant woman. Fpn - ferroportin, Fe-Tf - transferrin
The impact on fetal development and neonatal outcomes is less clear: maternal anemia is thought to be associated with a higher risk of perinatal and neonatal mortality, low birth weight, preterm birth, but a meta-analysis of randomized clinical trials on the effects of iron supplementation on perinatal outcomes showed only a modest effect on birth weight and an uncertain effect on the incidence of preterm birth [1,2]. However, some studies reported a 30% increase in the risk of fetal growth restriction for every 10 g/L decrease in hemoglobin before 20 weeks of gestation, and a decrease in hemoglobin <100 g/L significantly increased the risk of stillbirth and perinatal death. [10]. Postnatal iron deficiency during the first nine months is largely determined by the level of iron at birth, therefore, there is a direct relationship between maternal and fetal iron deficiency. The most common factors for the development of anemia in the fetus are acute maternal or placental hemorrhage and severe iron deficiency in the fetus (less than 90 g/l). Other factors leading to fetal iron deficiency include maternal hypertension, smoking, impaired glucose tolerance/diabetes mellitus with normal hemoglobin and ferritin levels, and multiple pregnancies [3].
Of greatest interest is the effect of iron deficiency on the neurocognitive functions of a child. Lack of iron in the mother in the third trimester leads to structural changes in the newborn's brain, including changes in the gray matter: neurons have a simpler structure, the functioning of mitochondria is disrupted. Iron-containing enzymes play an important role in the synthesis of fatty acids, which are an integral part of myelin, and therefore, iron deficiency at an early age changes the fatty composition of myelin and leads to hypo- and dysmyelination of the nervous system. Iron deficiency changes the expression of genes critical for the development and functioning of the hippocampus, and also changes the concentration of neurotransmitters dependent on iron-containing hydroxylases - tyrosine hydroxylase and tryptophan hydroxylase (dopamine, serotonin, norepinephrine), disrupts the functioning of their receptors and reuptake mechanisms, which inevitably leads to a number of psychoneurological disorders . [3,10].
Infants with non-anemic iron deficiency and a cord serum ferritin concentration <40 mg/L have impairments in recognition memory (the ability to recognize previously encountered people, events, objects), as do two-month-old infants with a cord serum ferritin concentration <76 mg/L. Impairments in recognition memory have also been observed before the age of 3.5–4 years in children with low neonatal iron levels, even if there was no deficiency in the postnatal period. Considering the fact that iron deficiency anemia in the mother is a predisposing factor for the development of iron deficiency in infants, the preconditions are created for impaired psychomotor development of the child. Iron deficiency in infancy and childhood is associated with slower information processing speed, impaired neurological reflexes, deterioration of motor function, and difficulties with socialization, which in turn creates the preconditions for the development of anxiety and depressive disorders in the future. Children aged 5–6 years have difficulties with learning, planning and concentration [3,10].
There are certain risks associated with the development of mental disorders in children born to mothers with iron deficiency anemia. Low iron levels in the preconception period are associated with a high risk of developing autism, and in the second trimester - with an increased risk of developing schizophrenia by 30% [3].
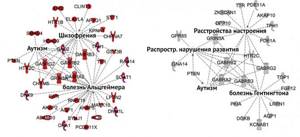
Figure 3 | Changes in gene expression in iron deficiency. On the left is the direct effect of deficiency on expression, on the right is epigenetic disturbances. [3]
As a rule, iron deficiency, along with other anemias, is diagnosed during a routine test of hemoglobin levels as part of a general blood test. Diagnosis of anemia in pregnant women has some peculiarities due to the variability of hemoglobin levels depending on the trimester. For the first trimester, the threshold value is Hb < 110 g/l, for the second – < 105 g/l, in the postpartum period – < 100 g/l. In a detailed blood test, erythrocyte indices are calculated: MHC (average amount of hemoglobin in an erythrocyte), MCV (average erythrocyte volume), MCHC (average hemoglobin concentration in an erythrocyte). Deficiency is characterized by a decrease in indicators, however, one should remember the physiological increase in MCV during pregnancy [1,6].
There are specific markers for iron: ferritin, various methods for assessing transferrin saturation: transferrin, the level of soluble transferrin receptors (sTSAT), %TSAT (transferrin saturation coefficient) and hepcidin - a peptide hormone that responds to excess iron. Hemoglobin concentration in reticulocytes also determines preanemia, iron deficiency and, therefore, can serve as an early biomarker of iron deficiency [3,5]. Serum iron and total serum iron-binding capacity are currently considered unreliable markers due to the influence of iron intake and diurnal fluctuations [6].
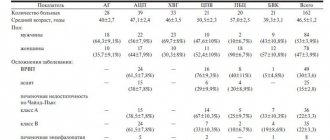
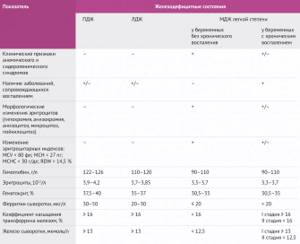
Table 2 | Diagnostic criteria for iron deficiency anemia in pregnant women. PID – pre-latent iron deficiency, LID – latent iron deficiency, MID – manifest iron deficiency. [7,8]
The most widely used hormone in pregnant women is ferritin, a marker of iron deposition in the mother's liver and placenta. It is ferritin that maintains a stable level of iron, removing from the depot the amount necessary for heme synthesis. A decrease in serum ferritin levels <30 μg/L is diagnostically significant. But in the case of concomitant systemic inflammation, ferritin can play a cruel diagnostic joke: the level of the marker increases, masking iron deficiency, and therefore a parallel study of C-reactive protein is recommended for differential diagnosis [1,3,6].
Transferrin is a protein responsible for the transport of iron in the blood plasma, responding to its deficiency by increasing its concentration. The sTfR value is proportional to the amount of transferrin. With a decrease in serum iron concentration, the concentration of sTfR increases simultaneously with transferrin. %TSAT is calculated by dividing the serum iron level by the total iron binding capacity of the serum and multiplying by 100. It reflects the percentage of iron binding sites on transferrin that are occupied by iron molecules. Serum iron concentration decreases in the early stages of deficiency, thereby reducing %TSAT. As deficiency worsens, transferrin concentrations increase to optimize the ability to bind iron for transport, further reducing %TSAT. All these changes occur before microcytosis or anemia [3].
The most interesting of the proposed markers is hepcidin, the measurement of serum concentrations of which may provide the most information about who should receive iron supplements and who should not receive them. Hepcidin is a major regulator of intestinal iron absorption and iron distribution from reticuloendothelial cells and is synthesized by the liver in response to iron levels and inflammation. Hepcidin is a negative regulator, meaning that high concentrations reduce intestinal iron absorption and promote iron sequestration, whereas low levels increase intestinal iron absorption and reticuloendothelial cell iron release. Patients with low hepcidin levels are likely to require iron supplementation and will respond to therapy, whereas patients with high hepcidin levels due to iron deficiency or overload do not require supplemental iron and will not absorb as much iron from food. Early pregnancy is characterized by very low hepcidin concentrations, indicating a state of negative iron balance and providing strong evidence that iron requirements are high during pregnancy. Thus, women whose iron levels are normal or borderline normal before pregnancy are prone to developing iron deficiency during pregnancy [3].
Sources:
- Pavord S. et al. UK guidelines on the management of iron deficiency in pregnancy //Br J Haematol. – 2021.
- Stanley L Schrier, Michael Auerbach. Treatment of iron deficiency anemia in adults//UpToDate, Feb. – 2021.
- Georgieff MK Iron deficiency in pregnancy //American journal of obstetrics and gynecology. – 2021.
- McMahon LP Iron deficiency in pregnancy //Obstetric Medicine. – 2010.
- McCann S., Perapoch Amadó M., Moore SE The Role of Iron in Brain Development: A Systematic Review //Nutrients. – 2021.
- Clinical recommendations “Blood-saving technologies in obstetric practice” 2014.
- Federal clinical guidelines “Diagnostics, prevention and treatment of iron deficiency conditions in pregnant and postpartum women” 2013.
- Dobrokhotova Yu. E., Bakhareva I. V. Iron deficiency anemia in pregnant women: prevention and treatment // Breast cancer. – 2021.
- Fisher AL, Nemeth E. Iron homeostasis during pregnancy //The American journal of clinical nutrition. – 2021.
- Benson CS et al. Iron deficiency anemia in pregnancy: A contemporary review //Obstetric Medicine. – 2021.