Frozen pregnancy is a pathological process that is characterized by the death of the fetus during intrauterine development. According to statistics, this occurs in almost 20-25% of all pregnancies, and this pathology is not very common among patients under the age of 30 and occurs in every second woman over 40-45 years of age.
Appointment with an obstetrician-gynecologist (doctor of the highest category) RUB 2,500.
Appointment with a hemostasiologist RUB 2,600.
All prices Make an appointment
In our International Hemostasis Clinic, pathology is treated by experienced gynecologists, candidates and doctors of medical sciences. Our specialists have extensive experience in the diagnosis, treatment and rehabilitation of such conditions. As a result, even after multiple frozen pregnancies, our patients manage to become pregnant and carry a healthy baby.
When and why is genetic analysis needed after a missed pregnancy?
The main goal of the study is to find out the reasons for the arrest of fetal development. Against the background of repeated miscarriages, it will indicate the factors that provoke recurrent miscarriage in the early stages.
If chorionic villus analysis is not performed after self-abortion, the situation may repeat during the next pregnancy. Having received the results of the cytogenetic analysis, the doctor will be able to develop treatment tactics or suggest using the services of a surrogate mother.
The analysis has diagnostic significance at a period of 10-12 weeks, when the ability of cells to divide can be preserved. MLC doctors recommend tests for those patients whose pregnancy has been diagnosed two or more times:
- at an early stage;
- a child is growing up with Down syndrome, Edwards disease, and other developmental defects;
- there are hereditary diseases in the parents (including on the father’s side) of the child;
- Karyotype abnormalities were established and a frozen pregnancy was diagnosed.
Why is a frozen pregnancy dangerous for a woman?
This condition requires mandatory treatment, as it can lead to a number of serious complications. Among them:
- Endometritis (inflammation of the inner lining of the uterus).
- Uterine bleeding.
- Peritonitis (inflammation of the peritoneum).
- Tissue sepsis (generalized infectious process).
- Maceration of the embryo (the process of intrauterine decomposition of the fetus).
- Chorioamnionitis (inflammation of the amniotic sac).
As you can see, the consequences are really severe and affect different systems of the body. Lack of treatment can have the most unfavorable prognosis for the patient.
How the research is carried out
For cytogenetic examination of the fetus, not only the fertilized egg released after self-abortion is suitable, but also any other biomaterial removed from the uterus during curettage. Karyotyping is only possible when analyzing living cells, therefore, if chromosomal pathologies are suspected, the patient should be under constant medical supervision.
During a frozen pregnancy, it is important not only to obtain the material on time, but also to store and transport it correctly. In our center, research is carried out in our own laboratory, so the problem of loss of sample quality, as a result of errors in identifying chromosomal pathologies, does not arise.
Indications
For the study, a fresh tissue sample from the miscarriage is taken, and the blood of the mother and father is provided for comparative analysis. Properly packaged samples with an official referral form are sent to a certified laboratory where testing is carried out. Or samples can be given directly at a clinic that has such a laboratory. Products of conception can be collected at home if a miscarriage occurs at home, but to properly collect the material, you need to get detailed instructions from your doctor.
Sorting of tissue samples is not necessary before sending to the laboratory, but this can significantly reduce the likelihood of contamination. Sorting involves removing large blood clots from the biopsy, rinsing it thoroughly with saline until no trace of blood remains, separating the villi from the sheath and placing them in a sample cup.
Testing will be useful for women experiencing pregnancy loss:
- over 35 years of age, the risks of random chromosomal abnormalities increase with age;
- with repeated miscarriages;
- after the first miscarriage, to know the risks;
- having children with congenital anomalies;
- who wants to know the reason for premature termination of pregnancy;
Also, when planning a pregnancy, the Natera laboratory offers:
| Test | What determines | When is it carried out? |
| PanoramaTM (non-invasive prenatal screening test, NIPT) | Several chromosomal aneuploidies, certain microdeletions | From 9 weeks of pregnancy |
| Horizon (carrier screening) | Determines the risk of transmitting certain genetic diseases to subsequent generations | Before pregnancy or early gestational age |
| Vistara | Detects serious diseases associated with 30 genes that standard NIPT does not test for. | From 9 weeks of pregnancy |
Candidates for embryo biopsy and PGD
Candidates for embryo biopsy and PGD include:
- Women over 34: Women are born with all the eggs they will ever have, and as a woman ages, her eggs are also affected by the aging process. Thus, the likelihood of conceiving chromosomally abnormal offspring increases with age. Overall, the risk of aneuploidy increases from 1 in 385 at age 30, to 1 in 179 at age 35, to 1 in 63 at age 40, and by age 45 there is a 1 in 19 chance of having an affected child. PGD with IVF has revealed that in reality, more than 20% of embryos in women aged 35 to 39 are aneuploid, and almost 40% of embryos in women over 40 are affected. Most of these embryos, if transferred into the uterus, either do not implant or result in miscarriage. This is considered the main reason for the low pregnancy and birth rates among women aged 40 years and older. Before the introduction of PGD, more embryos were transferred into the uterus to increase the chances of conception. Prenatal diagnosis after an IVF cycle is still strongly recommended as this confirms the prognosis of normal offspring. It is also possible that abnormal embryos may be mistakenly identified as normal and transferred to the uterus.
- Women with recurrent pregnancy loss (recurrent miscarriage): The man or woman of the couple may have abnormal chromosome packing, which can cause fatal abnormalities in some pregnancies but not others.
- Pairs with translocations: translocations are changes in the configuration of chromosomes in which chromosomes are attached to each other (Robertsonian) or sections of different chromosomes change places (reciprocal or reciprocal). About 1 in 900 people have Robertsonian translocations involving chromosomes 13, 14, 15, 21, 22. About 1 in 625 people have reciprocal translocations. To identify the presence of translocations, karyotyping of both partners can be performed. Couples with translocations may have recurrent pregnancy losses, or offspring with mental or physical problems. With a balanced translocation, when there is no additional or missing chromosomal material, and the break in the chromosome does not disrupt gene function, the person does not suffer. Carriers of balanced translocations may be affected by complex congenital malformations that may or may not be associated with an inherited disease. In an unbalanced translocation, in which there is or is no extra chromosome material, individuals will generally not be affected, although some will experience reduced fertility. However, there is a risk that eggs or sperm from such an individual may have unbalanced translocations, resulting in an unbalanced embryo. This can lead to implantation failure, recurrent miscarriage, or offspring with mental or physical problems.
- Couples with autosomal dominant disorders in which 50% of the embryos will be affected. Couples who have a family history of these disorders are either carriers or suffer from inherited diseases.
Couples with repeated IVF failures.
- Couples with a history of infertility may be able to determine the etiology, and therefore choose appropriate treatment.
- For couples at risk of inheriting a life-threatening disease, late-onset disease (Huntington's disease), it is preferable to plan, select appropriate treatments, or expedite the diagnostic process (for example, early diagnosis of breast cancer)
- Couples seeking offspring to produce HLA-matched stem cells for a suffering child with a terminal disease.
Methods used
To analyze the presence of genetic defects in an embryo, it is necessary to remove either the first polar body from the unfertilized egg and/or 1 or 2 cells from each embryo. This is called an egg or embryo biopsy and is usually done before fertilization occurs or 3 days after fertilization. Biopsy at the 6-10 cell stage does not have a negative effect on preimplantation development. At this stage, each cell has a full set of chromosomes. Usually only one cell is removed from the embryo, as it is expected to be the same as all other cells in the embryo. Sometimes it is necessary to remove a second cell from the embryo, for example, if no signal is detected in the first. FISH analysis is used to diagnose predisposition using the first and second polar bodies as indicators of the genetic status of the egg. A disadvantage of polar body analysis is that it does not take paternal aneuploidies into account.
Analysis of the biopsied cell uses one of two methods:
- Fluorescent in situ hybridization (FISH): The biopsied cell is fixed on a glass slide, heated and cooled, and its DNA is “tagged” with colored fluorescent dyes called probes (small pieces of DNA that correspond to the chromosomes being examined), one for each chromosome being detected. Currently, 8 of the 23 chromosomes can be identified. Once complete, the embryologist looks at the colors under a powerful microscope and is able, in most cases, to distinguish normal from abnormal cells. This process takes about a day. Normal embryos will either be transferred to the uterus on day 4 after egg retrieval, or will undergo extended culture and transferred on day 5 as blastocysts. The cells used for PGD are no longer viable and will not be returned to the embryo, but can be stored for future research.
- Polymerase chain reaction (PCR): a technique that increases the copy number of specific regions of DNA to produce enough DNA for analysis. DNA is double-stranded (except in some viruses), and the two strands are joined in a very specific way. The “building block” sequence of genes represents the specific order in which 4 different deoxyribonucleotides appear in a segment of DNA. The 4 components are: adenine (A), thymidine (T), cytosine (C), and guanine (G). The sequence of this 4-letter alphabet generates the composition of the gene. In this technique, the DNA is heated (denatured) to separate the 2 strands. Next, primers are added and the DNA is cooled so that double strands are formed again. Enzymes are then added to the cycles that can “read” the gene sequence, causing the DNA to multiply. PCR is used to diagnose gene-specific diseases, as well as to identify pathogenic viruses and/or bacteria, or in forensic science in connection with suspected crime.
All information is for informational purposes only. If you have any health problems, you need to consult a specialist.
Determining the chromosomal causes of molar pregnancy
A condition in which the embryo does not develop normally, and the chorionic villi form bubbles with liquid contents is called hydatidiform mole or molar pregnancy. The incidence of this rare complication is approximately 1:1000–5000 pregnant women. Women who become pregnant after the age of 40, with a previous miscarriage or molar pregnancy, and who are deficient in beta-carotene are at greater risk.
Hydatidiform mole poses a great threat to the health and life of a woman. It can be complete (80% of cases), characterized by the absence of embryonic tissue, or incomplete. The cause of complete molar pregnancy is the fertilization of a female reproductive cell that does not contain chromosomes. With further meiosis, the paternal chromosome set doubles, while chorionic villi only grow, and the embryo itself is not formed.
Incomplete hydatidiform mole is caused by fertilization of the female gamete by two sperm, which results in the appearance of extra chromosomes and the product of conception becomes nonviable. The cause is triploidy of male origin. In this condition, the tissues of the product of conception stimulate the production of human chorionic gonadotropin. High levels of hCG promote the formation of luteal cystic formations in the ovaries. 15–20% of cases of molar pregnancy are complicated by the development of a malignant neoplasm: when the products of conception are introduced into healthy maternal tissue, chorionepithelioma develops.
The hydatidiform mole must be removed surgically, after which hCG monitoring should be carried out. For chorionepithelioma, surgery and chemotherapy are performed. Timely detection of molar pregnancy is crucial for the patient. An ultrasound will only help to identify a hydatidiform mole; the Anora test will help determine the origin of triploidy. This is the only test that can detect the cause of a hydatidiform mole and prevent a woman from developing trophoblastic disease.
Using Anora testing, the number of analyzed samples is 7549, of which chromosomal abnormalities in 1268 samples are of fetal origin, in 1201 samples - with contamination. 95% of women who experienced a miscarriage and used the Anora test were satisfied with the results, because they learned the reasons for miscarriage and could more thoroughly prepare for the next pregnancy. Two thirds of patients who did not take up the opportunity to be tested would like to have a chromosomal test done.
How are genetic diseases inherited?
In the diagrams below, D or d represents the defective gene, and N or n represents the normal gene. Mutations do not always lead to disease.
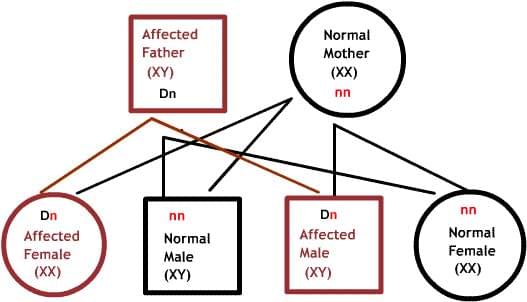
Dominant diseases:
One of the parents has one defective gene that dominates its normal pair. Since offspring inherit half of their genetic material from each parent, there is a 50% risk of inheriting the defective gene, and therefore the disease.
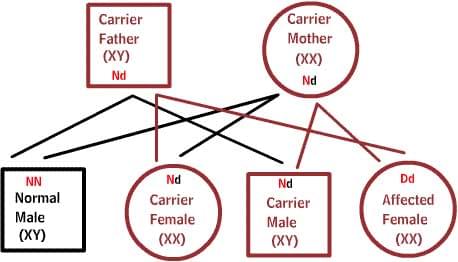
Recessive diseases:
Both parents are carriers of one defective gene, but at the same time have a normal gene pair. To inherit the disease, two defective copies of the gene are required. Each offspring has a 50% chance of being a carrier, and a 25% chance of inheriting the disease.
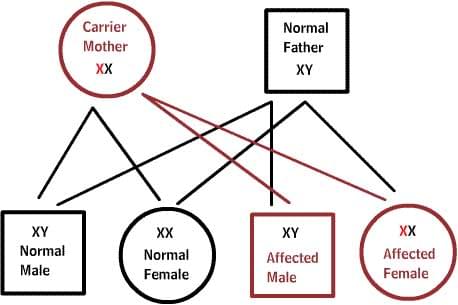
X-linked diseases:
Normal women have XX chromosomes, and normal men have XY chromosomes. Women who have a normal gene on one X chromosome are protected from a defective gene on their other X chromosome. However, a man lacks such protection due to the presence of only one X chromosome. Each male offspring of a mother who carries the defect has a 50% chance of inheriting the defective gene and disease. Each female offspring has a 50% chance of being a carrier, just like her mother. (in the picture below, X represents a normal gene and X represents a defective gene)
History of preimplantation genetic diagnosis (PGD)
The first live births after PGD were recorded in London in 1989. Two twin girls were born to five couples at risk of transmitting an X-linked disease. Currently, about 90% of abnormal embryos can be detected using genetic analysis or PGD methods. Not all chromosomal or genetic diseases can be detected by these procedures, as only a limited number of chromosomes can be diagnosed in one procedure. Numerous animal studies and some human studies show that fetal microsurgery (biopsy), which is necessary to remove cells, does not affect the normal development of the child. This procedure, however, has been performed on a relatively small number of patients worldwide, so the exact negative effects, if any, are unknown. Although many children have now been born around the world genetic testing No obvious problems were found in animal studies, and preliminary data in human embryos suggests the validity of this conclusion. In a study conducted at University College London, researchers recently examined 12 preimplantation embryos with a new technique that combines whole genome amplification (WGA) and comparative genome hybridization (CGH). As a result, significant chromosomal abnormalities were found in 8 of the 12 embryos studied. This may explain why people have at best a 25% chance of achieving a viable pregnancy per month when conceiving naturally.