The main component of the treatment of pelvic inflammatory diseases (PID) are antibiotics. Solving the issue of adequacy and timeliness of antibacterial therapy is the most pressing and is often life-sustaining [1, 2].
The complexity of resolving this issue is due to a number of factors:
— a variety of etiologically significant pathogens (mixed infections) of PID;
— the need to use broad-spectrum antibiotics or, more often, a combination of antibiotics;
— empiricism of initial therapy;
— often irrational use of antibiotics and the resulting increase in pathogen resistance [2, 3].
The variety of modern antibiotics provides sufficient opportunities for their choice, however, adequate therapy should be based primarily on the effectiveness and safety of the use of antibiotics proven in controlled clinical trials and, necessarily, data on the resistance of pathogens to them [1-3].
Based on the above, the purpose of our study was to study the sensitivity of the microbial flora of the cervical canal to antibacterial drugs in patients with PID.
Material and methods
A study was conducted of the sensitivity of the microbial flora of the cervical canal to antibacterial drugs in 501 patients with chronic PID aged 18 to 35 years, who contacted a gynecologist with complaints of pathological discharge from the vagina, aching pain in the lower abdomen and lumbar region, and lack of pregnancy. We included this category of patients in the main group (MG). The control group (CG) consisted of 84 patients aged 20 to 35 years, who contacted a gynecologist for a preventive examination and selection of a contraceptive method. The results of microscopy of smears from patients in the CG indicated that they had II degree purity of the vaginal contents.
Verification of the diagnosis in OH patients was based on data from clinical, laboratory (bacterioscopic, bacteriological examinations, PCR diagnostics, blood tests) and instrumental (ultrasound of the pelvic organs, computed tomography) examinations, as well as on the results of laparoscopy with morphological examination data. The results obtained indicate that all patients had PID, as well as cervicitis and vaginitis.
The bacteriological method (test systems, inoculation on 5% blood nutrient agar and accumulation media) included qualitative and quantitative isolation and identification of microorganisms from the discharge of the cervical canal in order to determine their sensitivity to antibiotics. All isolated microbial agents were tested for sensitivity to antibiotics using the disk diffusion method. The study was conducted before the start of taking antibacterial drugs. In addition, the interval after taking antibiotics (more than 6 weeks) was taken into account.
The results of the studies were subjected to statistical processing with the calculation of the arithmetic mean value (M),
errors of the average
(m)
and reliability of differences between indicators (p) taking into account the reliable probability according to the Student-Fisher test.
Results and discussion
Study of the results of bacteriological examination of the separated cervical canal (Table 1)
showed that in OG patients, compared with similar data in CG women,
E. coli
(p<0.01),
Staphylococcus
spp. significantly more often predominated.
(p<0.05), Ureaplasma urealyticum
(p<0.05),
Mycoplasma genitalium
(p<0.001).
Candida albicans
was detected only in OG patients.
The number of microbial associations (bacterial-bacterial and bacterial-fungal) during the examination of MG patients by bacteriological method was 29.94±2.05% (150 cases). Microbial associations included Ureaplasma urealyticum
- 42 (8.38±1.24%),
E. coli
- 36 (7.19±1.15%),
Candida albicans
- 32 (6.39±1.09%),
Staphylococcus haemolyticus
- 17 (3 .39±0.81%).
The results of determining the sensitivity of the microbial flora of the cervical canal to antimicrobial drugs in patients with chronic PID are presented in table. 2.

E. coli haemolyticus
(Gram-negative bacteria) has a high degree of sensitivity to macrolides (azithromycin and josamycin) and fluoroquinolones (norfloxacin, ofloxacin) compared to inhibitor-protected penicillins (amoxicillin clavulanate) and cephalosporins (cefotaxime, cefofloxacin, cefoperazone). The resulting difference was statistically significant: 1) azithromycin/amoxicillin-clavulanate (p<0.001); azithromycin/cefotaxime (p<0.001); azithromycin/cefofloxacin (p<0.001); azithromycin/cefoperazone (p<0.001); 2) josamycin/amoxicillin clavulanate (p<0.001); josamycin/cefotaxime (p<0.001); josamycin/cefofloxacin (p<0.001); josamycin/cefoperazone (p<0.001); 3) norfloxacin/amoxicillin clavulanate (p<0.001); norfloxacin/cefotaxime (p<0.001); norfloxacin/cefofloxacin (p<0.001); norfloxacin/cefoperazone (p<0.001); 4) ofloxacin/amoxicillin clavulanate (p<0.001); ofloxacin/cefotaxime (p<0.001); ofloxacin/cefofloxacin (p<0.001); ofloxacin/cefoperazone (p<0.001).
Among cephalosporins, E. coli haemolyticus
was detected to ceftazidime (p<0.001).
In other representatives of gram-negative flora (Klebsiella, Proteus mirabilis)
a high degree of sensitivity was detected to norfloxacin (p<0.001) and ceftazidime (p<0.001).
In addition, high sensitivity in E. coli haemolyticus
(p<0.01) and in
Klebsiella
(p<0.001) was noted to gentamicin.
Analysis of the sensitivity of gram-positive cocci showed (see Table 2),
that high sensitivity (more than 50%), compared with that to other macrolides, to azithromycin was noted in
Staphylococcus
spp.
(azithromycin/clarithromycin, p<0.05; azithromycin/roxithromycin, p<0.001; azithromycin/spiramycin, p<0.001) and Streptococcus
spp.
(azithromycin/josamycin, p<0.001; azithromycin/clarithromycin, p<0.001; azithromycin/roxithromycin, p<0.001); to josamycin - in Staphylococcus
spp.
(josamycin/clarithromycin, p<0.05; josamycin/roxithromycin, p<0.001, josamycin/spiramycin, p<0.001) and Enterococcus faecalis
(josamycin/azithromycin, p<0.001, josamycin/roxithromycin, p<0.001).
Among gram-positive cocci the highest sensitivity (see Table 2)
to fluoroquinolones was noted in
Enterococcus faecalis
to moxifloxacin: significantly higher than to other fluoroquinolones (p<0.001), as well as to azithromycin (p<0.001) and josamycin (p<0.05).
Sensitivity Staphylococcus
spp. to ofloxacin (p<0.001) was similar to that to azithromycin and josamycin, but the highest compared to sensitivity to moxifloxacin (p<0.01), sparfloxacin (p<0.001), ciprofloxacin (p<0.001).
Highest sensitivity Streptococcus
spp. to drugs from the fluoroquinolone group was fixed to ofloxacin; however, this indicator was significantly lower than sensitivity to azithromycin (p<0.05).
High sensitivity Staphylococcus
spp.
and Streptococcus
spp.
was noted to amoxicillin clavulanate. This indicator for Staphylococcus
spp.
was significantly higher than the sensitivity index to azithromycin (p<0.001), josamycin (p<0.001), and ofloxacin (p<0.001). Sensitivity of Streptococcus
spp. to amoxicillin clavulanate was as high as to azithromycin, but significantly exceeded the same indicator for josamycin and other macrolides (p<0.001), as well as for ofloxacin and other fluoroquinolones (p<0.001).
According to our studies, the sensitivity of gram-positive cocci (see Table 2)
to cephalosporins was significantly low compared with that to macrolides (p<0.001), fluoroquinolones (p<0.001), and inhibitor-protected penicillins (p<0.001).
Studying the sensitivity of representatives of the Mollicutes
(see Table 2),
we obtained data indicating that the sensitivity of
Ureaplasma urealyticum
was significantly high to josamycin (p<0.001), spiramycin (p<0.001), doxycycline (p<0.001) according to compared with that of other macrolides, fluoroquinolones.
Sensitivity of Mycoplasma hominis
was significantly high for josamycin (p<0.001) compared with this indicator for other macrolides, fluoroquinolones, and doxycycline.
Thus, we found that according to the bacterial study, microbial associations amounted to 29.94±2.05%. Microbial associations included Ureaplasma urealyticum, E. coli, and Staphylococcus haemolyticus.
From a practical point of view, it is important to lower the diagnostic threshold for PID to initiate empirical treatment, since any delay in diagnosis and treatment increases the incidence of late complications [1, 4].
According to a number of researchers, treatment regimens for PID should ensure the elimination of a wide range of possible pathogens. At the same time, one should assume the possible resistance of microorganisms to traditional antibiotics. During hospitalization, it is advisable to start treatment with the parenteral route of drug administration and then switch to oral forms [5-7].
Our data indicate that the highest sensitivity is in gram-negative bacteria, gram-positive cocci, and representatives of the class Mollicutes
was noted for azithromycin and josamycin compared with other groups of antibacterial drugs. The results of the study should be taken into account when empirically prescribing antibacterial drugs, especially in gynecological hospitals, in patients with acute course or exacerbations of PID.
conclusions
1. The highest sensitivity is in gram-negative bacteria, gram-positive cocci, representatives of the class Mollicutes
was noted for azithromycin and josamycin compared with other groups of antibacterial drugs.
2. Priority is parenteral administration of azithromycin with subsequent correction and selection of individual antimicrobial therapy after obtaining results determining the sensitivity of microbial flora to antibiotics.
How is a culture taken from the cervical canal?
The analysis is carried out by microscopy of the material on days 4-5 of the menstrual cycle. For a smear test, the cervix is exposed using a speculum. Using a sterile swab or brush, collect mucus from the surface of the epithelium, turning it clockwise several times, trying not to damage the membrane.
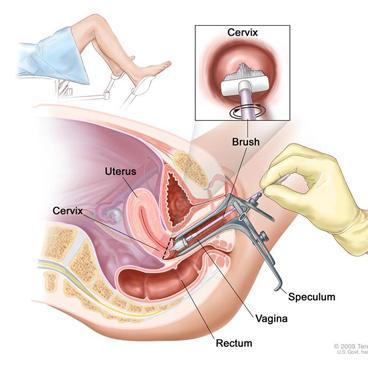
This is how they take a smear from the cervical canal - click to enlarge
The brush is removed and the resulting material is distributed in an even layer on a glass slide, avoiding drying. The glass is placed in an individual bag and sent to the laboratory.
The entire duration of the procedure for collecting material for research is 15 minutes.
If transportation of the material is required, then it is carried out only at a temperature not exceeding 20 degrees in a sealed bag - a refrigerator. In laboratory conditions, opportunistic material is placed in an environment favorable for their reproduction.
Each type of bacteria requires individual conditions and time of reproduction. The results at the end of the process are recorded by laboratory staff. The interpretation is carried out by a gynecologist.
Note! Tank culture from a cervical analysis does not reveal the presence of infections such as: herpevirus, ureaplasma, mycoplasma, chlamydia (penetrating into cells and affecting the nucleus). This type of microorganism can be detected by PCR diagnostics (polymerase chain reaction).
How to prepare for a cervical smear?
Proper preparation for a painless procedure ensures reliable results. The doctor writes a referral to the patient and talks about the nuances that need to be taken into account before taking the test.
A gynecologist takes tests on a pregnant woman. Sexual contact is avoided for several days before the procedure. Do not take medications or contraceptives.
If a few days before the scheduled date of delivery, vaginal examinations were carried out using a speculum, then it is advisable to postpone the procedure. It is forbidden to do douching, which distorts the vaginal microflora. To maintain hygiene, use regular boiled water without using detergents.
Before visiting the treatment room, wash only in the evening . 1-2 hours before the appointment, refrain from urinating. The procedure does not cause discomfort or pain to the patient.
Culture of flora with determination of sensitivity to antibiotics
This is a microbiological study that makes it possible to determine the qualitative and quantitative composition of the microflora of the biomaterial under study, including the identification of high-titer opportunistic microorganisms and pathogenic microorganisms, and their sensitivity to antibiotics.
When microbiological methods detect microorganisms that constitute normal microflora or opportunistic microorganisms in a titer less than the diagnostic one, sensitivity to antibiotics and bacteriophages is not determined, since this amount is not significant and does not require treatment with antimicrobial drugs.
Aerobic microflora is studied.
Synonyms Russian
Bacteriological culture of flora with determination of sensitivity to antibiotics.
English synonyms
Culture, routine. Bacteria identification and antibiotic susceptibility testing.
Research method
Microbiological method.
What biomaterial can be used for research?
Single portion of urine, urogenital swab (with prostate secretions), sputum, oropharyngeal swab, breast milk, nasopharyngeal swab, ejaculate, ear discharge, conjunctival swab, nasal swab, synovial fluid, cervical swab, urethral swab , oral abscess discharge, pleural fluid, cerebrospinal fluid, bronchial washings, bile, exudate, biopsy, contents of the gallbladder.
How to properly prepare for research?
- It is recommended to drink a large volume of water 8-12 hours before collecting sputum.
- Avoid taking diuretics for 48 hours before urine collection (in consultation with your doctor).
- Women are recommended to take a urogenital smear or urine before menstruation or 2 days after its end.
- Men should not urinate for 3 hours before submitting a urogenital smear or urine test.
- Do not brush your teeth on the day of taking biomaterial for research.
General information about the study
Normal human microflora is a collection of microorganisms that inhabit the skin and mucous membranes. The largest number (about 40%) lives in the gastrointestinal tract, the rest - on the skin, pharynx, pharynx, genitourinary system, etc. Normal microflora is divided into permanent (accounts for up to 90% of microbes present in the body), facultative ( less than 10%) and random (no more than 0.5%).
Based on their ability to cause infectious diseases, microorganisms are classified into non-pathogenic (not causing disease), opportunistic (normally they can be released in small quantities and under certain conditions they actively multiply, leading to inflammation) and pathogenic (they are causative agents of infectious diseases and are not part of the normal microflora are detected).
Bacteriological research (culture on the flora) allows you to determine the qualitative and quantitative composition of the microflora of the clinical material being studied, including the identification of pathogenic microorganisms. When opportunistic microorganisms are detected in high titers or pathogenic microorganisms, their sensitivity to antibiotics and bacteriophages is determined.
What is the research used for?
- To identify the causative agent of an infectious disease.
- To select rational antimicrobial therapy.
- To evaluate the effectiveness of the therapy.
When is the study scheduled?
For inflammatory diseases of various localizations (with the exception of inflammatory bowel diseases).
What do the results mean?
Reference values for various types of microorganisms depend on their location (point of collection of biological material).
What can influence the result?
Previous antifungal or antibacterial therapy.
Also recommended
- Culture of flora with determination of sensitivity to bacteriophages
- Bacteriological examination of clinical material on a VITEK bioMerieux analyzer with determination of sensitivity to antibiotics
Who orders the study?
Therapist, general practitioner, pediatrician, surgeon, ENT, pulmonologist, urologist, gynecologist, dermatovenerologist, ophthalmologist.
Literature
- Chernecky S.S. Laboratory tests and diagnostic procedures / S.S. Chernecky, B. J. Berger; 5th ed. – St Louis: Saunders Elsevier, 2008. – 1232 p.
- Gill VJ, Fedorko DP, Witebsky FG The clinician and the microbiology laboratory. In: Principles and practice of infectious disease / G. L. Mandell, Bennett J. E., R. Dolin (Eds) ; 6th ed. – Churchill Livingstone, Philadelphia, PA 2005. – 2701 p.
- Levinson W. Medical microbiology and immunology: examination and board review (Lange medical books series) / W. Levinson; 8th ed. – NY: McGraw-Hill, 2004. – 654 p.