Scientists at Washington University School of Medicine in St. Louis have found that you can get rid of chronic cystitis by making your urine less alkaline. This refutes the previously existing claim that high acidity of urine protects against infections.
It turned out that for the most common causative agent of the disease, E. coli, highly acidified urine is a suitable habitat. Under such conditions, the siderocalin protein, which has a detrimental effect on pathogens, is destroyed.
pH standards
Normal acidity is a rather broad term that does not give a complete picture of the state of the body at the moment due to the influence of numerous factors.
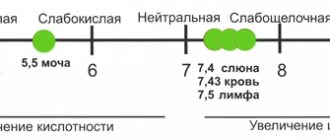
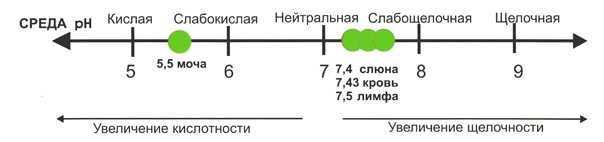
There are generally accepted indicators, going beyond which is characterized as the presence of pathology. For urine, the pH will range from 5.0 to 7.0. Short-term fluctuations in acidity from 4.5 to 8.0 can be considered normal if they are short-term and there are no alarming symptoms such as polyuria, oliguria or pain when urinating.
Also, pH values fluctuate depending on the time of day, degree of physical activity, individual characteristics of the body or diet. For example, in the morning the pH is 6-6.5, and in the evening the acidity rises to 7. In addition, the ratio of the liquid secreted to the liquid drunk is of great importance.
Optimal acidity levels in men may be higher than in women due to a higher percentage of muscle mass, as well as a dietary pattern that involves consuming more meat products. Be that as it may, the optimal generally accepted acidity value for adults is in the range from 6.3 to 6.5.
In women during breastfeeding, this figure can rise to 7.8. As a result of the high level of metabolism for newborn children, the acidity numbers will be completely different. The average baby has a urine pH level of 5.4 to 5.9 units, and for premature babies it is 4.8 to 5.4.
How and why to measure the pH of urine and saliva?
It is necessary to pay attention to changes in the pH level of the internal environment of the body
The main regulators of the acid-base balance in the human body are carbon dioxide and hydrogen ions H+. Hydrogen plays a major role in the formation of acids and alkalis, its concentration must be within strict limits controlled by the body. When the number of hydrogen ions deviates from normal, malfunctions occur in the functioning of enzyme systems and functional proteins, which leads to the development of various diseases. It is very important to pay attention in time to changes in the pH level of the internal environment of the body and, if necessary, take urgent measures.
We control the pH of the body by measuring the pH of urine and saliva
It is easier to monitor the body's pH level by measuring the pH of urine and saliva. The urine reaction of an adult when consuming mixed food is slightly acidic or neutral (pH in the range of 5.0–7.0, average 6.0). Depending on the nature of the food, the urine reaction can range from 4.5 to 8.0. An acidic urine reaction is observed with an overload of meat foods, an alkaline reaction is observed with a vegetable diet. In addition, the acidity of urine changes in many diseases of the body.
The alkalinity of urine increases when drinking mineral waters during vomiting and diarrhea, chronic urinary tract infections, cystitis and inflammatory diseases of the bladder (except for cystitis caused by Escherichia coli or Mycobacterium tuberculosis). Acidity increases with diabetes mellitus, tuberculosis of the kidneys and bladder, renal failure, with fever, fasting, kidney stones, hypokalemia and hypochloremia, infusion of large amounts of isotonic sodium chloride solution, in children with exudative diathesis.
In addition to the pH of urine, when diagnosing a health condition, the pH of saliva is measured, which depends on the rate of salivation. Typically, the acidity of mixed human saliva is 6.8–7.4 pH, but with high salivation rates it reaches 7.8 pH. In children, on average, the acidity of mixed saliva is 7.32 pH, in adults – 6.40 pH. A low acidity value (6.2–6.0) of saliva not only indicates a disruption in the functioning of the body, but also leads to demineralization of tooth enamel with the appearance of erosion of hard tissues and the formation of cavities in them - caries.
It is convenient to measure the pH of biological fluids with an electronic pH meter
Quite often it is recommended to measure the pH of saliva and urine using indicator paper, but indicators of this type are inaccurate, and in the case where the urine is intensely colored, they may generally give incorrect results. For the body, deviations in pH by a tenth of a unit are very significant. In addition, a single measurement of the pH of urine and saliva is not very informative - for diagnostic purposes, several measurements must be taken per day, which means that the cost of disposable indicator strips does not allow much savings.
The best option for measuring the pH of biological fluids is electronic pH meters. SIMVOLT company offers for such purposes the use of convenient portable pH meters of the EZODO 5000, EZODO 6000, EZODO 7000 series. It is enough to immerse the device in a glass with biological fluid and the pH value will appear on the display. In the case of saliva, it is necessary to collect a sample of liquid in a small container of such a volume that the glass electrode of the device is completely immersed. After use, you just need to rinse the electrode with distilled water and put the device in a storage case.
For preventive purposes, it is recommended to measure urine pH 3 times a day for 3 days, saliva pH - twice a week, 2-3 times a day. The best time to determine the pH level is 1 hour before a meal or 2 hours after a meal.
Using portable electronic pH meters, you can independently monitor the acidity of your body, which will allow you to promptly prevent the possibility of developing various diseases. Electronic pH meters can also be used in clinical studies and medical tests - this will allow you to get accurate results in the shortest possible time
Rules for using test strips
Does beets affect the color of urine: is it good or bad?
Urine pH can be easily determined using reagent test strips at home.
Preparation for the procedure:
- When removing strips, do not touch the indicator part of the plate with your fingertips.
- Collect the morning urine sample in a sterile container, after performing an external hygienic toilet of the genitals. Do not take the test during your period.
- Stir the urine, eliminating possible sediment.
- Conduct diagnostics at temperatures from 15 to 25 degrees. Lower environmental figures may cause false readings.
- Do not use urine collected more than 2 hours ago. The error in values in this case is up to 50%.
- Do not use the strip more than once.
- Do not keep the plate in urine for more than the time specified in the instructions to avoid obtaining incorrect test results.
- Do not use devices that have expired.
- Avoid exposure of ultraviolet rays to the diagnostic color scale located on the tube.
Carrying out the procedure:
- Carefully remove the strip from the plastic box.
- Stir the urine, eliminating the sediment of salts.
- Immerse the plate in the container for 1-3 seconds.
- Remove the indicator, remove excess sorbent liquid, or tap the strip on the edge of the container.
- Place the test on a dry surface with the reagent side up.
- Assess the value of the indicators after 1-3 minutes. according to the color scale: orange – 4.9-5.0; saturated yellow – 6.0; pale yellow – 6.5; light green – 7.0; pale green – 7.5; emerald – 8.0; swamp green – 8.5.
- The waiting time for results should not be exceeded. Analysis values can change significantly under the influence of air and ultraviolet radiation.
Acidity is determined once every 3-4 days. It is advisable to conduct the study at the same time (immediately after getting up in the morning), since the numbers vary greatly throughout the day. If the indicators shift over 2 weeks towards acidification or alkalization, you must consult a general practitioner and undergo a comprehensive medical examination
Reasons for deviations from the norm
Causes of poor urine analysis
Alkalosis and acidosis are abnormal conditions for the body. This means that there are disturbances in the body due to physiological or pathological reasons. The first category includes:
- irrational physical activity;
- peculiarities of eating behavior (unbalanced diet);
- excessive passion for alcoholic beverages;
- incorrect treatment with certain medications.
In this case, changing your diet and lifestyle will help restore normal pH. An impaired acidity reaction may indicate the development of acute and chronic diseases. However, pathologies are not necessarily associated only with the urinary system.
Acidification of urine
A low pH means the urine is highly acidic. Most often, acidic urine accompanies metabolic disorders, in particular, people with diabetes suffer from this feature. And also a shift of values to the left is observed in the following cases:
- an excess of saturated acids, protein foods and fats in the daily menu (meat, baked goods, butter), as well as a passion for protein diets;
- electrolyte imbalance associated with deficiency of potassium (hypokalemia) and chlorine (hypochloremia);
- inflammation of the bladder walls (cystitis);
- damage to the tubular system of the renal apparatus (pyelonephritis);
- extrapulmonary infection with Koch's bacillus (nephrotuberculosis);
- metabolic disorders (in particular, excessive formation or intake of acids);
- formation of ketone bodies (acetone) in the urine as a complication of diabetes mellitus;
- trauma to the pancreas, with further formation of a pancreatic fistula;
- postoperative syndrome, after ureterosigmoidostomy (surgery on the ureters);
- chronic stool disorder (diarrhea);
- excessive consumption of alcoholic beverages;
- CRF (chronic renal failure);
- inability of the kidneys to remove uric acid (resulting in the development of gout);
- urolithiasis and nephrolithiasis (the presence of urate stones in the organs of the urinary system);
- overdose of calcium and ammonium chloride medications;
- hypervitaminosis of ascorbic acid;
- intoxication of the body;
- adrenal dysfunction.
- intense physical activity (sports training).
A decrease in acidity values can accompany oncohematological diseases and sepsis.
Alkalinization of urine
If the pH is elevated, it means that the urine is predominantly alkaline. A high level of alkali indicates the development of acute conditions, the progression of chronic diseases, and poor nutrition. The following are the causes of alkalinization of urine:
Strips for acetone in urine
- immunoinflammatory damage to the glomeruli of the kidneys (glomeruli), otherwise glomerulonephritis;
- chronic renal failure;
- vegetarianism and veganism, plant-dairy diets with minimal consumption of protein products and fats;
- abuse of table mineral water with a high alkaline content;
- decreased functionality of the adrenal glands to produce hormones (hypocorticism and hypoaldosteronism);
- electrolyte imbalance due to increased potassium concentration (hyperglycemia);
- overactive parathyroid gland (hyperparathyroidism);
- hyperacid gastritis (inflammation of the gastric mucosa, accompanied by increased acidity of gastric juice);
- pyelonephritis and cystitis of bacterial etiology (origin);
- dehydration (dehydration), due to repeated vomiting and diarrhea (symptoms of body intoxication);
- long-term treatment with neurotransmitters (Epinephrine hydrotartrate, Adrenaline);
- incorrect use of nicotinic acid preparations;
- exacerbation of gastric ulcer.
An alkaline reaction can be caused by inflammatory dental diseases, due to a violation of the acid-base balance of the oral cavity. If there are stable deviations in the acid level (regardless of its increase or decrease), it is necessary to examine the kidneys and take a general and biochemical blood test. If pathological changes are not detected, you should adjust your diet, physical activity and give up alcoholic beverages.
Increased vaginal acidity and its causes

The normal vaginal pH ranges from 3.8 to 4.5. If the indicator is less than 3.8, then we are talking about an increase in the level of vaginal acidity.
Factors influencing changes in vaginal acidity include:
- Using inappropriate intimate hygiene products. For daily washing, you need to use special gels and foams that have a neutral pH level. If you use simple soap for this purpose, there is a chance of disturbing the pH balance of the vagina.
- Long-term treatment with antibiotics. Antibiotics not only destroy pathogenic microorganisms, they also reduce the amount of valuable microflora. Therefore, antibiotic treatment should be carried out under the strict supervision of a physician.
- Excessively frequent and intensive intimate hygiene. If you douche and vaginal douche too often, you can disrupt the pH environment of the vagina.
- Stress, living in unfavorable environmental conditions, chronic fatigue, lack of sleep, poor nutrition and a general decrease in the body's defenses, which can manifest itself in the form of changes in pH levels.
- Hormonal surges and disruptions. It is known that pH-metry of the vagina during pregnancy, before menstruation, during menopause or at the beginning of puberty gives different results.
Expert opinion
The balance of estrogen and progesterone in the body of a healthy woman of reproductive age is optimal for maintaining a normal level of vaginal acidity. If the level of estrogen decreases for any reason, there is a disruption in the process of glycogen production, leading to a decrease in the number of lactobacilli.
Obstetrician-gynecologist of the highest category Oksana Anatolyevna Gartleb
Methods for determining urine pH at home
Urine test strip test
You can independently determine the acidity of urine in several ways:
- Litmus paper;
— Magarshak method;
— Bromothymol blue indicator;
— Visual indicator test strips.
The reagent of litmus paper is a dye based on azolythmine and erythrolythmine. When conducting a study, blue and red litmus paper is immersed in the urine, and the results of the analysis are deciphered by their color. If both papers change color, then the reaction is amphoteric; both papers do not change color - neutral. If the blue paper turns red but the red paper does not change color, the reaction is acidic. When red paper turns blue and blue does not change, an alkaline reaction of urine is observed.
This method allows you to determine in which direction the acidity of the urine has shifted, but in order to find out the exact pH value, liquid indicators are needed.
Determination of urine acidity using the Magarshak method is carried out using an indicator consisting of a mixture of alcohol solutions of neutral red and methylene blue. The approximate pH value of urine is determined by the shade that the urine acquires after adding an indicator to it.
Bromothymol blue indicator is also used to determine the acidity of urine. Examination of urine in this way shows only approximate acidity.
Determining the pH of urine using test strips is a simple and convenient option for home use. The test strip must be dipped into a container of urine, and then compared with a color scale, the colors on which correspond to the pH of the urine.
No method of self-determination of urine acidity can replace laboratory testing in a medical facility.
Indicators that are assessed during general clinical urine analysis
The study evaluates:
- color
– various shades of yellow are acceptable; - transparency
. Cloudiness of urine indicates the presence of pathological elements in it - leukocytes (indicate purulent processes), bacteria, precipitated salts (phosphates), etc. - specific gravity (density)
- depends on the concentration of dissolved substances (urea, uric acid, creatinine, salts. Reduced specific gravity can be a sign of pyelonephritis, increased - glomerulonephritis, diabetes mellitus, cardiovascular failure; - urine reaction (pH)
– assessment of acidity. There are weakly acidic, neutral and slightly alkaline reactions. It should be taken into account that this indicator can be affected by the use of certain vitamins and medications, as well as the nature of the diet: a meat diet increases acidity, a vegetarian diet, on the contrary, makes the reaction slightly alkaline. An increased pH value (alkalinization) may indicate kidney disease, inflammatory processes of various localizations and some other diseases. A low pH value (high acidity) is characteristic of dehydration of the body, which is typical in particular for febrile states, severe diarrhea, and may also indicate diabetes mellitus or some other pathologies; - Protein in urine
is not normally detected. Identified during inflammation and other pathological conditions; - glucose in urine
is normally absent. Glucose in the urine during pregnancy (in the second or third trimester) is gestational diabetes, which usually resolves immediately after delivery. It occurs due to increased stress on the body with high hormone activity. Glucose in the urine during pregnancy is a reason to optimize your lifestyle and diet, but first you need to make sure that the appearance of sugar in the urine is not pathological; - bile pigments - bilirubin and urobilinogen
; - blood cells (leukocytes and erythrocytes), epithelial cells, foreign agents (bacteria, fungi, parasites) and other indicators.
pH (reaction) of urine
Reference values (or norm) of urine pH (urine reaction): the norm with a normal diet is slightly acidic (pH 5.0 - 7.0). Fluctuations in pH (4.5 - 8.5) depend on the composition of food taken, medications taken, and kidney function. Eating meat causes an acidic reaction, while eating plant foods causes an alkaline reaction in urine.
An increase in pH above normal is called alkalosis, and a decrease is called acidosis. Eating a large amount of plant foods shifts the pH of urine to the alkaline side (pH 7.5), eating meat, on the contrary, shifts it to the acidic side (pH 5 – 5.5),
Urine pH
A decrease in pH to 5 (shift to the acidic side) of urine is caused by active physical work, diabetes, fasting, dehydration, fevers, impaired renal function, taking ascorbic acid, methionine.
An increase in pH above 7 occurs when drinking excessive amounts of mineral water, when there is blood in the urine, when the bladder is inflamed, taking adrenaline, bicarbonates.
Establishing the acidity value of urine is extremely important in the treatment of urolithiasis - if the stones are represented by tripelphosphates, then alkaline acidity is extremely undesirable, since it can cause their formation. If the stones are represented by urates, then during treatment it is necessary to maintain the alkaline acidity of the urine to dissolve such stones
Urine pH determination
- Urine pH is reduced (alkaline reaction) when:
- low protein or plant-based diet
- taking alkalizing medications such as sodium bicarbonate
- respiratory and metabolic alkalosis
- renal tubular acidosis (as a result of disruption of the ability of the proximal tubules to reabsorb bicarbonates, which leads to alkalinization of urine and acidification of the blood)
- chronic urinary tract infections (bacteria break down ammonium)
- Urine pH is increased (acidic) when:
- eating a lot of meat
- taking acidifying medications
- respiratory and metabolic acidosis
- diabetes and ketosis
- fasting, fever
- gout
- potassium deficiency in the body (potassium is reabsorbed in exchange for hydrogen ions, which leads to acidification of urine)
Determining the pH of urine has not only diagnostic value, but also, which is especially important, allows you to more correctly explain its other indicators. For example, the absence of blood cells (erythrocytes, leukocytes) in diseases of the kidneys and urinary tract, occurring with hematuria and leukocyturia, can be explained by the alkaline reaction of urine, in which these elements are quickly destroyed
The reaction of urine affects the activity and reproduction of bacteria, as well as the effectiveness of antibacterial therapy.
Clinical interpretation of urinary pH is only relevant when urinary pH measurements are correlated with other information about the patient's health, or when a diagnosis has been made and urinalysis results indicate the course of a disease.
In other words, urine pH has little clinical significance, but when combined with other symptoms and laboratory values, it can provide important information. The determination of urine pH can be especially indicative against the background of the following pathology: urinary tract infection, renal tubular acidosis, respiratory acidosis, respiratory alkalosis, metabolic acidosis, metabolic alkalosis, drug monitoring, Fanconi syndrome, urolithiasis. The pH ratio of urine and blood in some pathological conditions
| Ph urine | blood ph | Possible pathology |
| Sour | Sour | Diabetes, febrile conditions, fasting, kidney failure, kidney tuberculosis, leukemia, etc. |
| Alkaline | Alkaline | Cystitis, pyelitis, hematuria, after vomiting and diarrhea, during the resorption of exudates and transudates, when taking soda and mineral waters |
| Alkaline | Sour | Hyperchloremic acidosis, renal tubular acidosis, chronic urinary tract infections - bacterial decomposition of nitrogen-containing substances in urine to ammonia |
| Sour | Sour | Hypokalemia, treatment of alkalosis with intravenous infusion of large quantities of NaCl (paradoxical aciduria) |
Herbal preparation CANEPHRON in urological practice
Treatment of kidney and urinary tract diseases with medicinal plants has a long history. However, there was a period when doctors used them little. In recent years, the “well forgotten old” has regained its position.
Medicinal plants are increasingly used as therapeutic agents prescribed by internists, nephrologists and urologists.
Their advantages over synthetic drugs are obvious in most cases. Firstly, there are no complications or unwanted side effects. Secondly, there is a wide scope for maneuvering, which is provided by a rich selection of plants with different types of action. It would be irresponsible to claim that medicinal plants can cure any disease, that is, they are a panacea.
The priority of their use is dictated by the nature of pathological changes, the phase of the disease, and the presence of complications.
One of the rules of herbal medicine can be formulated as the principle of its indication and priority. Guided by this principle, the role of medicinal plants at a certain stage of the disease should be determined. It can be basic, parity and auxiliary. The second principle of herbal medicine is the principle of its individuality.
We want to share our observations about the herbal preparation canephron (Bionorica, Germany), which appeared in our country not so long ago, but has been produced in Germany since 1934. This period of use of the drug in itself already indicates its effectiveness. Canephron is a combination drug of plant origin. It contains centaury (Herba Centaurii), lovage (Radix Levistici), rosemary (Folia Rosmarini), rose hips (Fructus Cynosbati).
The substances included in the drug have an antiseptic, antispasmodic, anti-inflammatory effect on the genitourinary tract, reduce the permeability of the capillaries of the kidneys, have a diuretic effect, improve kidney function, and potentiate the effect of antibacterial therapy (see table).
| Medicinal plants and their spectrum of action | |||
| Medicinal origin | centaury | Lovage | Rosemary |
| Bitters, phenolcarbolic acids | Essential oils, phthalides | Rosmarinic acid, essential oils, flavonoids | |
| Diuretic | + | + | + |
| Antispasmodic | — | + | + |
| Anti-inflammatory | — | — | + |
| Antibacterial | + | + | + |
Centaury herb contains alkaloids, flavonoid compounds, bitter glycosides, and phenolic acids.
Rosehip peel contains a large amount of ascorbic acid, organic acids, a lot of sugar, pectic acids, organic acids (citric and malic), as well as carotene.
Lovage contains essential oils, phenolcarboxylic acids, and phthalides.
Rosemary contains rosmarinic acid, essential oils and flavonoids.
Various types of effects of canephron on the body are due to its constituent essential oils (lovage, rosemary), phenolcarboxylic acids (rosemary, lovage, centaury), phthalides (lovage), bitters (centaury).
The diuretic effect of canephron is due to the combination of various points of application of medicinal substances. Essential oils dilate the blood vessels of the kidneys, which improves blood supply to the renal epithelium, and also influence the function of the epithelium of the renal tubules. This manifests itself mainly in a decrease in the reabsorption of Na+ ions and the corresponding amount of water. The diuretic effect of phenylcarboxylic acids is explained by the osmotic effect. The principle of action is that when they enter the lumen of the renal tubules, they create high osmotic pressure (these drugs are not subject to reverse absorption), and the reabsorption of water and Na+ ions is significantly reduced.
Due to the removal of excess fluid and sodium salts from the body, canephron helps lower blood pressure. Herbal diuretics act somewhat weaker and slower than modern synthetic drugs, but they are more gentle.
As is known, the main signs of inflammation are associated with the action of inflammatory mediators (bradykinin, prostaglandins, histamine, serotonin, etc.). The anti-inflammatory properties of canephron are mainly provided by rosmarinic acid and are associated with suppressing the synthesis of inflammatory mediators or slowing down their release and activation.
All medicinal plants that make up canephron contain substances (phenolcarbolic acids, essential oils, etc.) that have an antimicrobial effect. It is important to emphasize the wide antimicrobial spectrum of medicinal plants and their activity against microflora resistant to synthetic drugs. The advantage of these medicinal plants is the combination of antimicrobial and anti-inflammatory properties, which is especially valuable for chronic processes in the urinary tract. It has been established that canephron enhances the excretion of uric acid salts. This side of the action is only partly related to the diuretic effect and is quite specific. Increasing the secretion of uric acid prevents the loss of crystals in the urinary tract, the growth of existing stones and the formation of new ones. It was also noted that this drug alkalinizes the urine, if it is sharply acidic in urate nephrolithiasis, and keeps it within 6.2–6.8, which also prevents the formation of urate stones.
The antispasmodic effect of canephron is due to flavonoids.
The pharmacologically proven effect of canephron on the tubular apparatus of the kidney clearly shows that the excretion of protein in the urine due to previously suffered pathological processes damaging the tubular apparatus is significantly reduced.
We used Canephron in the urological clinic of the I.M. Sechenov Moscow Medical Academy in various groups of patients.
Treatment with this drug was carried out in 68 patients: 18 patients suffering from chronic pyelonephritis, 15 patients with chronic cystitis, 17 patients with nephrolithiasis. In 18 patients, Canephron was used after shock wave lithotripsy.
During treatment, in 16 patients with chronic pyelonephritis, leukocyturia disappeared after a three-week course. In 15 patients with chronic cystitis, dysuria disappeared on the 10th day after treatment; on the 19th day, urine analysis returned to normal.
We also found that in patients suffering from urate nephrolithiasis, and there were 17 of them, on the 5th day the pH of urine became within the range of 6.2–6.8, and this is one of the main conditions in the treatment of urate stones and the prevention of their formation . It was also noted that the daily excretion of uric acid increased in all of these patients.
Thus, these results indicate that Canephron should be more widely used in patients suffering from urate nephrothiasis for prevention and treatment.
Thanks to the antispasmodic and diuretic effect of canephron, in patients of all groups with various diseases, daily diuresis increased to 2.2–2.5 l, while there was no change in the Na+ concentration in the biochemical blood test.
This suggests that this drug is a mild herbal diuretic.
It is known that the sooner the stones pass after shock wave lithotripsy, the better. The use of Canephron in this group of patients showed that in all patients taking the drug, the fragments passed within 4-5 days. In the control group, stones passed within 5-14 days.
Thus, the herbal preparation canephron has a wide spectrum of action, although in our study we examined only its antimicrobial, anti-inflammatory, antispasmodic and diuretic activity.
However, judging by the available data, canephron is widely used for renal failure, proteinuria, and interstitial nephritis. Canephron is indicated for a large group of patients suffering from chronic inflammatory diseases: pyelonephritis, cystitis. It should be prescribed to patients with urate nephrolithiasis, as well as for the passage of small stones after shock wave lithotripsy.
No side effects or complications were observed when using this drug for 4, 6, 8 weeks.
The drug can be recommended for both children and adults; it is also prescribed to pregnant women suffering from inflammatory diseases of the urinary system.
Reasons for changes in urine acidity
Most metabolic products are excreted from the body through the kidneys, so you need to understand that acidity is due to the influence of many factors.
By and large, acidity is a dynamic value that differs from person to person and even changes from one person to another depending on the food consumed, medications taken, lifestyle or time of day. A change in the pH of urinary sediment can occur towards acidification or towards alkalization.
Acidification
Urine acidification is a condition in which the pH becomes less than 5.0. This may occur due to a change in diet, increased physical activity, or pathology of the urinary system.
There are a huge number of diseases that contribute to changes in the acidity of urine. Basically, the pH drops to 5 in diabetes. An acidic urine reaction occurs under the following conditions:
- metabolic acidosis;
- Diabetes mellitus is characterized by a significant change in the composition of urine, not only in terms of a decrease in acidity, but also in the form of an increase in the amount of glucose;
- fever;
- Gout is a common rheumatological disease, the characteristic symptom of which is the acidic environment of urine. The disease is caused by a violation of purine metabolism, as a result of which a large amount of uric acid begins to accumulate in the body;
- leukemia;
- eating low carbohydrate foods;
- An increase in urine acidity may be caused by drugs that increase diuresis. This means that such medications are only allowed to be taken in short courses;
- infectious diseases of the urinary system caused by Escherichia coli or mycobacteria;
- chronic renal failure;
- eating foods high in protein. In addition to meat, acid-boosting foods include white bread, fish and cheese;
- sepsis;
- treatment with ascorbic acid in a dosage of more than 2 g per day significantly increases the pH of urine and also increases the risk of developing urolithiasis;
- pathologies of the digestive system.
When urine acidity is elevated for more than 10 days, this is an important laboratory indicator indicating a metabolic disorder or a decrease in the filtration function of the glomerular apparatus of the kidneys.
Also, a slight decrease in the pH level of urinary sediment occurs in newborns. Acidic urine in a newborn is completely physiological and should not cause concern to parents. As the child gets older, the acidity of the urine will level out.
Alkalinization
Alkalinization of urine is a condition in which the pH level becomes more than 7. Alkalinity in the urine can be detected with regular consumption of lactic acid or plant products, as well as with bacterial and metabolic diseases. The reasons for such deviations may be the following factors:
- chronic bacterial urinary tract infection. Microbes are able to ferment nitrogen-containing compounds to ammonia, which leads to an increase in pH;
- hyperkalemia;
- adrenal hormone deficiency;
- renal tubular acidosis;
- metabolic and respiratory alkalosis;
- passing bloody urine (hematuria);
- increased levels of phosphate-containing compounds in the urine;
- drinking large amounts of mineral water;
- a diet containing large amounts of plant foods, black bread, milk;
- inflammation of the walls of the urinary tract (cystitis, urethritis);
- postoperative period.
In severe pathological processes, chronic renal failure often occurs, leading to alkalization of urine. This is caused by both congenital (primarily wrinkled kidney, pathology of the renal vessels) and acquired (glomerulonephritis, pyelonephritis, diabetic kidney) causes.
Also, temporary alkalization of urine can be caused by intravenous administration of a solution of buffered soda. It is administered in emergency cases accompanied by significant acidification of the blood (sepsis, liver failure, ketoacidotic coma).
Clinically, an increase in pH levels is manifested by general weakness, diffuse headache, nausea and vomiting.
Diet for cystitis
Those who want to make their urine less acidic naturally should exclude from their diet:
- fruits that can oxidize urine - citrus fruits, sour apples and plums;
- sour juices and drinks containing acidulants;
- Fatty foods that increase uric acid levels in the body.
Since meat and offal also “acidify” urine, their use for cystitis should be limited.
It is allowed to eat various vegetables, grain porridges, legumes, and alkaline medicinal mineral water such as “Essentuki”.
Rules for collecting urine for analysis
To obtain reliable data, the patient must know how to properly collect urine for general analysis.
- On the eve of urine collection, you should not eat fatty, spicy, fried, or sweet foods. If there are a lot of products, they will affect the result.
- You should not take new medications for 3-4 days. The exception is drugs that affect a woman’s life and health. But the attending physician and laboratory assistant are warned about them.
- Immediately after waking up in the morning, they begin to collect urine, but before that you should not eat food or drink large amounts of water.
- Urine is collected in a sterile container, which is purchased at a pharmacy. Cans, bottles and other contaminated containers should not be used. Even if you clean and disinfect them yourself, foreign substances will remain inside. They influence the data obtained.
- Before emptying the bladder, close the vaginal opening with a cotton swab. This is necessary so that foreign bacteria and pregnancy discharge do not get into the sample.
- For a general clinical examination of urine, the first portion of liquid is flushed down the toilet, and the middle portion is collected in a container. After collection, the container is closed with a lid.
- The urine sample is immediately taken to the laboratory. But there are times when it can be collected early in the morning when the clinic is closed. Then the liquid is placed on the door of the refrigerator. Do not place it in the freezer or the coldest parts of the refrigerator. The sample is left in the cold for no more than 2-3 hours. After this time, uncontrolled biochemical processes will begin that change the result.
As the uterus grows, the condition of the internal organs changes. The liver, kidneys, and digestive tract are compressed. Therefore, clinical tests are constantly taken during pregnancy in order to promptly identify abnormalities.
What is acidification and alkalization of the body, blood, urine: signs and symptoms
What is acidification and alkalization of the body, blood, urine: signs and symptoms Danger to the body is posed by acidic foods that we are accustomed to consuming without paying attention to the norm. Few people know how harmful these products are to our health. So, what is acidification and alkalization of the body, blood, urine?
- The blood of a healthy person has a slightly alkaline reaction: 7.35-7.45. If your blood test results are higher, this is a disease; lower, too.
- Most people suffer precisely from acidification of the body - acidosis.
- It is easy for a raw foodist with alkalosis to be cured by adding acidic foods to the menu, but for a meat eater with acidosis it is much more difficult to recover.
Signs and symptoms if acidity increases greatly:
- Immunity decreases - a person begins to get colds often.
- Bones become brittle - the body uses a lot of calcium to alkalize.
- The activity of good enzymes decreases - you feel lethargic and constant fatigue.
- The body retains water - the limbs, face, or whole body swell.
The body does not like excessive oxidative processes, and it responds by refusing to function in specific organs and systems. A lot of effort is spent on processing acidic products. The more you use them, the more energy you use. Nutrients such as calcium, potassium, sodium and iron are consumed.
How to increase vaginal acidity and why might this be necessary?
From a medical point of view, it is more correct to talk not about increasing the pH level of the vagina, but about its restoration and normalization. To do this, you need to make an appointment with a gynecologist, who will select the appropriate therapy. The main thing in such cases is not to self-medicate, as this can further aggravate the situation.
Treatment of low and high vaginal acidity usually includes correction of local immunity, combating pathogenic flora and normalizing the lactobacilli population.
Properties of urine
Urine is a yellow physiological fluid that is formed during the life of the body. Its main function is the removal of metabolic products, regulation of osmotic pressure and ionic composition of the blood. During the day, 800–1500 cm³ of urine is released, this is the norm for a healthy person. With the development of any diseases, indicators may change up or down. Diuresis depends on a person’s physical activity, ambient temperature, body weight, and humidity.

Urine is produced in the kidneys while filtering blood. The tubules regulate the absorption and excretion of ions, then the fluid flows through the ureters into the cavity of the bladder and is excreted through the urethra. In healthy people, urine has a light yellow color; when red blood cells, cholesterol and other pathological components appear, its shade changes, a sediment forms, and an unpleasant odor appears.
Urine consists of more than 90% water, the rest is salts and breakdown products of protein compounds. With the development of diseases, impurities of sugar, blood, ketone bodies, protein, leukocytes, oxalic salts, lactic acid, and red blood cells may be found in the urine. Electrolytes are released along with urine: calcium, sodium, potassium, magnesium, sulfates, as well as hormones, enzymes and vitamins.
Antibiotics are not omnipotent: the causative agents of cystitis will survive this
The content of the article
Chronic cystitis is an insidious “sore” that is aggravated by hypothermia, overheating, wearing synthetic underwear and other reasons. The inflammatory process is caused by many microorganisms, the most unpleasant of which is E. coli, which lives in the intestines. Most often, microbes enter the bladder through the urethra due to insufficient hygiene of the genital organs.
In recent years, the cause of cystitis caused by E. coli has become the fashion for thong swimsuits. Along wet “ropes”, like paths, the sticks easily move from the anal area to the urethra and bladder.
Antibiotics were previously used to fight the disease, but recently their effectiveness has decreased. The reason is self-medication, leading to insensitivity of the pathogen to drugs. In the last 10-15 years, there have been more and more cases of chronic cystitis that are difficult to treat with conventional drugs. Therefore, scientists have to look for an alternative.
Preparing to test urine for an acidic environment
The most objective indicators are obtained by examining morning urine.
Before collecting it, it is necessary to carry out hygiene procedures for the genitals. It is better to submit urine for analysis in sterile containers purchased at a pharmacy no later than 2 hours after collection. If the shelf life is longer, alkalization or an increase in acidity may occur.
Urine should be delivered to the laboratory at a temperature of at least 10 °C. At lower temperatures, a precipitate forms, making research difficult.
Before submitting urine for analysis, it is not recommended to:
- Take medications and food at least 8 hours before the test.
- Physical exercise.
- Eating fried, smoked, pickled foods.
- Drinking alcohol, coffee, or foods containing pigment that affects the color of urine.
How is urine pH determined in the laboratory?
A general urine test evaluates not only the characteristics of the fluid, but also the composition of the sediment. The doctor is obliged to prescribe a test to diagnose the provoking change in the composition of urine, and also again after recovery from an infectious disease.
The PH of urine will show endocrine disorders, the presence of sand and stones in the kidneys. Low urine acidity (below 5.5) causes the formation of uric acid stones. A urine pH of 5.5-6.0 is fraught with the appearance of oxalate formations, and more than 7 - phosphate kidney stones. A laboratory method such as titration gives a more accurate acidity result.
Preparatory measures and rules for collecting urine are of great importance for the accuracy of the result. For an accurate value, you need an average portion of urine, which is collected in the morning on an empty stomach. Be sure to wash the external genitalia thoroughly before collection. The biomaterial is collected in a cleaned glass container or plastic container, which must be delivered to the laboratory within the next few hours.
Two or three days before the urine test, you should not drink alcoholic beverages, herbal teas, or medications, especially diuretics. It is not recommended to submit urine for testing during menstruation. A day before the urine test, brightly colored fruits and vegetables are excluded from the diet.
How to cure cystitis
The main thing when cystitis occurs is not to self-medicate, but to consult a urologist who will prescribe the necessary tests and treatment. Often the exacerbation is so severe that it is simply impossible to cope with it with diet and urine-alkalinizing drugs alone.
If you notice the symptoms of cystitis - pain and pain when urinating, you need to:
- Take a general urine test, which will also show acidity;
- Perform an ultrasound of the bladder or a comprehensive ultrasound of the pelvis;
- Take a smear for infections.
This set of tests and examinations is needed to identify the cause of cystitis. The disease does not occur on its own: it is a consequence of at least one of the factors:
- Activities of pathological bacteria;
- The occurrence of stones in the bladder;
- Problems with the organ: changes in wall thickness, fistula, tumors of the bladder or neighboring organs.
Normal values
The acidity of urine is determined by the following features:
- age category of the patient (adults and children);
- gender (women and men);
- time of day (morning and evening);
- nutritional characteristics (predominance of protein, plant or dairy products in the diet);
- the presence of chronic diseases (endocrine, urinary system, gastrointestinal tract).
In women, the acid-base balance is affected by pregnancy and breastfeeding. Normal pH levels range from 5 to 7 units. According to accepted laboratory standards, slightly acidic urine is considered healthy. The optimal value in this case is pH 6.
Children's indicators
In an infant, the acidity of urine depends on the feeding habits (artificial formula or natural feeding), as well as on the time of birth relative to the duration of pregnancy (term or premature).
| Babies born at term | Premature babies | Artificials | Breastfed babies |
| from 5.4 to 5.9 units. | from 4.8 to 5.4 units. | from 5.4 to 6.9 units. | from 5.6 to 6.0 units. |
Unstable pH in a newborn baby does not cause concern, provided that there are no genetic pathologies or congenital developmental abnormalities. Normalization of the level occurs on days 3–4. In children and adolescents up to adulthood, the normal pH of urine falls within the range of 6.5 to 7.5 units.
Level in adult men and women
The acidity level in adult men is affected by muscle mass (MM); in women, it is influenced by the perinatal period and lactation. During pregnancy, a slight excess of reference values is allowed.
| Men | Women | |||
| average MM | high MM | not pregnant | pregnant women | nursing |
| 6,3–6,5 | 6,5–7,2 | 6,0–6,5 | 5,0–8,0 | 6,5–6,8 |
Deviations of a short-term nature are observed at night and in the morning (before breakfast). The pH level can drop to 5.2 units. in the morning, and increase in the evening to 7 units. Such “swings” do not pose any danger. A constant acidity value in the morning and evening, which does not go beyond the normative limits, does not apply to pathologies. If during the day the reaction remains stably acidic or stably alkaline, there is a need for additional examination.
Additionally
Acidification of urine (acidosis) occurs at pH levels of 5 and below. Uric acid, salts of phosphoric and oxalic acids interact with liquids in which the acidity level is normal or slightly elevated. When the pH decreases to 5 units or less, they turn into sediment.
A consistently high level of acidity provokes the formation of uric acid urate stones with a soft structure. In people of reproductive age, the location of stones is usually the kidneys and ureter; in elderly patients and children, urolithiasis (urolithiasis) affects the bladder. In addition, acidified microflora is a favorable environment for the proliferation of pathogens.
Escherichia coli is usually the most active. Escherichia coli is a gram-negative bacterium that can cause not only severe poisoning. Excessive spread of E. coli can provoke: inflammation of the meninges (meningitis), pneumonia, hemolytic-uremic syndrome, systemic blood infection (sepsis).
Alkalosis (alkalization of urine) corresponds to values exceeding pH 7. In this case, there is a high probability of the formation of phosphates (alkaline stones) in the renal pelvis and calyces. A feature of stones of phosphate origin is their accelerated growth. If the diagnosis is not made in a timely manner, the only way to get rid of phosphates is through surgery.
An alkaline environment inhibits the body's immune functions, which promotes the penetration and rapid development of bacterial infections of the bladder and kidneys. pH values of 8 and higher are recorded for bacteriuria (the presence of bacteria in the urine). The most dangerous infections include varieties of Streptococcus (staphylococcus):
- Staphylococcus aureus – Staphylococcus aureus;
- Staphylococcus epidermidis – epidermal staphylococcus;
- Staphylococcus saprophyticus is a saprophytic staphylococcus.
Colonization of staphylococcal microorganisms in the urine leads to the development of bacterial cystitis and bacterial pyelonephritis.
An important factor that can have a modulating effect on the formation of kidney stones is urine pH. This indicator is used both to assess the risk of stone formation and to monitor the result of anti-relapse treatment in patients with urolithiasis (UCD) [1,2]. Normally, urine is slightly acidic with an average pH of about 6.0, although its acidity in a healthy person can vary from 4.5 to 8.0 [3,4]. The acidity of urine can change in diseases such as urothelial tumors, metabolic disorders and urolithiasis [5,7]. In urolithiasis, it has been established that urine acidity can influence various stages of stone formation, including crystallization, growth, aggregation and stone retention in the urinary tract [8-10]. In addition, urine pH is an important factor that can promote the formation of the solid (crystalline) phase and have a litholytic effect against urinary stones [11,12]. The formation of some types of urinary stones (oxalate, phosphate, urate, cystine) largely depends on the pH of the urine [1,8,12]. Alkaline urine pH promotes the formation of phosphate stones, while acidic urine pH is associated with the formation of urate and cystine stones [13,14].
However, the acidity of urine is not the only factor that determines the ability of urine to form stones, that is, its lithogenic properties. An increase in urine lithogenicity occurs when the excretion of various ions and substances that can affect stone formation is impaired [15]. A number of different ions and substances are known, impaired excretion of which in the urine can increase the lithogenic potential of urine, which, apparently, is the result of the mutual influence of both metabolic and physicochemical factors on each other, including urine pH [15].
Previously published studies examined the dependence of the formation of stones of a certain chemical nature on the intensity of exposure to such metabolic risk factors for ICD as calciuria, uraturia, phosphaturia and magnesiumuria [16,17].
Purpose of the work: taking into account the important modifying effect of urine pH on lithogenesis, to study the influence of this physicochemical factor on the severity of the studied metabolic risk factors for stone formation, as well as on the frequency and risk of formation of urinary stones of various metabolic types.
MATERIALS AND METHODS
636 patients with urolithiasis (274 men and 362 women aged 16 to 77 years) were examined. The mineral composition of urinary stones and metabolic parameters were determined using methods described previously [16], the pH of morning urine was determined using an AutionMaxAX-4280 analyzer (Arcray, Japan). The urine pH values were divided into seven ranges, which were used as factors in the analysis of variance (ANOVA module of the Statistica v.10 program), and the values of daily excretion of calcium, uric acid, phosphate, magnesium and the percentage of certain minerals in urinary stones were used as dependent variables. The classification of urinary stones was carried out according to the predominant mineral component (more than 50% of the total mineral base) [18-20].
RESULTS AND DISCUSSION
The results of the analysis show that the pH value of urine has a noticeable effect on the incidence of stone formation of various metabolic types in patients with urolithiasis (Fig. 1).
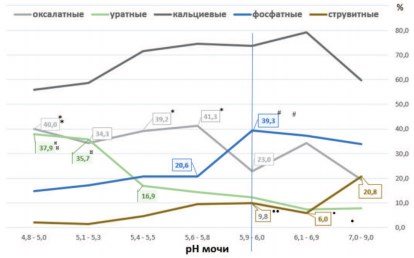
Fig.1. Frequency of detection of the main types of stones (in%) at different urine pH values. *p<0.01 vs. pH 5.9-6.0 (for oxalate stones); #p<0.02 vs. pH 5.9-6.0 (for phosphate stones);¤p<0.003 vs. pH 5.4-5.5 (for urate stones); •p<0.015 vs. pH7.0-9.0,••p<0.03 vs. pH7.0-9.0 (for struvite stones)
It can be noted that with an increase in the urine pH value and a shift in the urine reaction to the alkaline side, there is a slight decrease in the frequency of detection of calcium oxalate stones in patients with urolithiasis (Fig. 1). Correlation analysis carried out across seven pH ranges indicates such a trend (r = -0.739; p = 0.0576). However, a more detailed analysis reveals the following features. With an increase in pH from 5.9-6.0 to pH 7.0-9.0, a statistically significant increase in the frequency of detection of oxalate stones is not observed. A shift in urine pH to the acidic side (from 5.9-6.0 to 4.8-5.0) causes an increase in the incidence of oxalate stones among patients with urolithiasis by 70.4-79.6% (p < 0.01) .
Wewellite is known to be the main mineral phase of calcium oxalate urinary stones. As was established in this work, the proportion of wewellite in oxalate stones with a predominance of the oxalate component of more than 70% was 80.2%, and wedellit - 11.3%. In stones represented by 100% oxalate mineral phase, the proportion of wewellite was even higher - 92.2%, and wedelite - only 7.8%.
Analysis of the oxalate content in urinary stones as a function of urine pH confirms the fact that cases of oxalate urolithiasis increase with increasing urine acidity (Fig. 2). A shift in urine pH to the acidic side (from 5.9-6.0 to 4.85.0) leads to an increase in the proportion of wewellite in the stones (Fig. 2A). The dynamics of vedellite accumulation in stones is of the opposite nature, indicating the accumulation of this mineral in stones when urine is alkalized from pH 5.1-5.3 to pH 6.1-6.9 (Fig. 2B). As noted, wavellite represents almost entirely the oxalate component of stones, so the proportion of oxalate in urinary stones also increases with increasing urine acidity (Figure 2C).
Rice. 2. Proportions (in %) of wewellite (A), vedellite (B) and oxalate mineral component (C) in urinary stones at different urine pH values. Data are presented as M±m (means with standard errors of the means indicated by vertical lines) op<0.02vs. pH 5.9-6.0; oop<0.07vs. pH 5.9-6.0; #p<0.068vs. pH 5.1-5.3; ##p<0.02vs. pH5.9-6.0
The data obtained are consistent with the results of experimental studies. It was found that crystals of calcium oxalate monohydrate (wewellite) formed most actively at acidic pH values (starting from pH 4.0) and least intensively at alkaline values (at pH 7.08.0) [21]. At the same time, differences in such parameters as the volume of crystals formed, their number and mass differed by 2-3 times or more.
Thus, a decrease in urine acidity below pH 6.0 can increase the risk of developing oxalate urolithiasis. Obviously, this lithogenic effect is realized through the modifying influence of changes in urine pH on other metabolic risk factors for stone formation, and primarily on the intensity of calciuria.
An analysis of the influence of urine pH on the excretion rates of some lithogenic substances and ions showed that an increase in calcium excretion in the urine is observed when the urine pH decreases from 5.9-6.0 to 5.4-5.5, reaching a maximum at a pH of 5. 6-5.8 (Fig. 3). It is interesting to note that it is at these pH values and maximum values of calciuria that the maximum activity of oxalate stone formation is observed in patients with urolithiasis (Fig. 1). The high frequency of detection of oxalate stones in patients remains almost constant, ranging from 40.0% to 41.3%, with a further increase in urine acidity up to pH 4.8-5.0, despite a significant decrease in calciuria by 1.42 times ( Fig. 1.3).
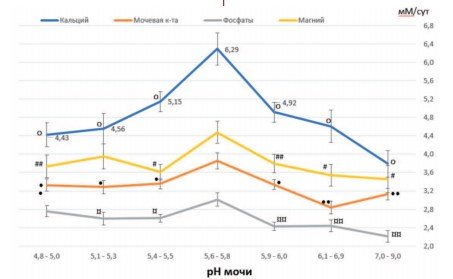
Fig.3. Excretion rates of calcium, uric acid, phosphates and magnesium (in mM/day) at different urine pH values. Phosphate excretion values are given as mM/day x10-1. Data are presented as M±m (means with standard errors of the means indicated by vertical lines). op<0.005 vs. pH 5.6-5.8; #p< 0.02vs. pH5.6-5.8;##p<0.057vs. pH5.6-5.8; •p< 0.009vs. pH 5.6-5.8;••p< 0.0006vs. pH 5.6-5.8;¤p< 0.03 vs. pH 5.6-5.8; ¤¤p< 0.003 vs. pH 5.6-5.8
Thus, in increasing oxalate lithogenesis, a decrease in urine pH (below 6.0) plays a more significant role than increased calcium excretion.
Indeed, in the pH range from 5.9-6.0 to 5.6-5.8, an increase in oxalate lithogenesis is aggravated by calciuria (from 4.92 to 6.29 mmol/day), the reduction of which should be the goal of anti-relapse therapy. At pH values from 5.6-5.8 to 5.4-5.5, metaphylaxis of oxalate urolithiasis should be more aimed at correcting disturbances in urine pH, since under these conditions even a decrease in the level of calciuria does not significantly affect the reduction in the frequency of formation of oxalate stones (Fig. 1.3).
Unlike oxalate stones, the formation of phosphate stones, represented by carbonate apatite, increases with alkalinization of urine. With maximum values of calciuria and phosphaturia observed at pH 5.65.8, there is a low frequency of detection of phosphate stones from carbonate apatite (20.6%) in patients with urolithiasis (Fig. 1.3). The highest frequency of phosphate urolithiasis is observed with an increase in pH to 5.96.0 (39.3%, p<0.02, Fig. 1). The observed decrease in the levels of calcium and phosphate excretion indicates the leading role of urine alkalization in the formation of phosphate stones (Fig. 1,3). This suggests that the main goal of metaphylaxis of this type of stones should be to maintain the patient’s urine pH in the range from 5.65.8 to 5.4-5.5, and not to combat concomitant calciuria and phosphaturia, which have virtually no effect on phosphate lithogenesis.
The role of urine alkalization in the formation of carbonate apatite stones is illustrated in Figure 4. As the pH of urine increases and its alkalization, a progressive increase in the proportion of carbonate apatite (phosphate component) is observed both in the stones as a whole (Fig. 4 A) and in mixed oxalate-phosphate calcium stones (Fig. 4 B). Similar results were obtained in other studies [22,23].
Rice. 4. Content of carbonate apatite (in %) in urinary stones (A) and in mixed calcium oxalate-phosphate stones (B) at different urine pH values. Data are presented as M±m (means with standard errors of the means indicated by vertical lines). op<0.03vs. pH 4.8-5.0; oop<0.05 vs. pH5.6-5.8; $p< 0.035vs. pH4.8-5.0; $$p< 0.02vs. pH5.6-5.8
The influence of shifts in urine pH on the frequency of detection of calcium (mixed oxalate phosphate) stones in patients is not entirely clear. As noted, the formation of the oxalate or phosphate component in these stones is regulated by multidirectional shifts in urine pH. Despite the fact that a decrease in the frequency of detection of calcium stones is observed when urine pH is below 5.4-5.5 and above pH 6.1-6.9 (Fig. 1), maintaining pH within these limits should hardly be recommended for metaphylactic purposes , since under these conditions there is a danger of activation of urate or struvite stone formation, respectively.
The frequency of detection of cases of urate urolithiasis is closely related to changes in urine pH. The percentage of detection of urate stones in patients gradually increases as the urine pH decreases from alkaline values of this indicator (7.0-9.0) to acidic values (4.8-5.0), (Fig. 1). Correlation analysis indicates a fairly close inverse relationship between changes in urine pH in seven selected pH ranges and the incidence of urate stones (r = -0.815, p = 0.0255).
A particularly noticeable increase in the incidence of urate urolithiasis is observed when urine pH decreases below 5.4-5.5 (Fig. 1). At the same time, the frequency of detection of urate stones increased more than 2 times - from 16.9% to 35.737.9% (Fig. 1, p < 0.003).
Urate urolithiasis is usually associated with persistently low urine pH, which is observed in almost all patients with urate stones, despite the fact that urate excretion is not impaired in most patients [24,25].
As noted in this work, the frequency of detection of cases of urate urolithiasis did not increase with maximum uric acid excretion (Fig. 1, pH value 5.6-5.8), which confirms the leading role of low urine pH values in urate lithogenesis compared to increased excretion of urates [26,27]. Indeed, at pH 5.0, uric acid can form crystals at a very low molar concentration - about 2 mmol/l, while at pH 5.9-6.0, a concentration of 4 mmol/l or more is required for its crystallization [28] .
The dissociation constant of uric acid (pKa) corresponds to 5.5 [27,29]. Therefore, when urine pH is less than 5.5, uric acid becomes insoluble and, depending on its concentration in the urine, crystals of anhydrous acid or uric acid dihydrate are formed [30]. This dependence is illustrated by Figure 5, where the majority of urate stones, represented by both pure 100% urates and stones with a predominance of the urate component (>60% of the composition), are formed at acidic urine pH values.
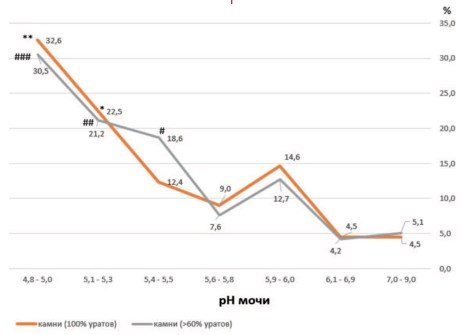
Rice. 5. Predominant formation of urate stones (detection frequency in %) at acidic urine pH values (below pH 5.6-5.8). Legend: *p < 0.02 vs. pH 5.6-5.8; **p < 0.02 vs. pH 5.6-5.8; (for stones containing 100% urate); #p < 0.02 vs. pH 5.6-5.8; ##p < 0.003 vs. pH 5.6-5.8; ###p < 0.00005 vs. pH 5.6-5.8 (for stones containing more than 60% urate)
Noteworthy is the coincidence of high frequencies of oxalate and urate urolithiasis at urine pH below 5.5 (Fig. 1). It is known that crystals of anhydrous uric acid can serve as heterogeneous centers (nucleants) for the formation of crystals of calcium oxalate monohydrate (wewellite), especially when urine is oversaturated with oxalate and calcium under conditions of deficiency of crystal formation inhibitors [31,32]. The ability of uric acid to act as a heterogeneous nucleant for wavellite crystals is much higher than that of mucin (glycoprotein) or cellular detritus, but lower than that of some calcium salts [31]. Moreover, uric acid was found as a minor component in the central part of vellite stones, the core [33], which indicates its participation in oxalate lithogenesis and explains the high frequency of detection of oxalate stones in patients with urine pH below 5.5, including wavellite stones, which make up more than 90% of all oxalate stones.
It is clear from this that with acidic pH values of urine, the relative risk of the formation of oxalate and urate stones is increased, in contrast to alkaline pH values of urine, and the risk of formation of urate stones is 2.4 times higher than oxalate stones (Table 1).
Table 1. Relative risk of urinary stone formation at identified reference urine pH values
| pH<5.9 | pH>6.0 | R.R. | 95%CI | P | ||
| Oxalate Stones | Total stones | Oxalate stones | Total stones | |||
| 138 | 358 | 38 | 144 | 1,47 | 1,09-1,99 | 0,0123 |
| pH<5.4 | pH>5.5 | R.R. | 95%CI | P | ||
| Urate Stones | Total stones | Urate stones | Total stones | |||
| 61 | 165 | 35 | 329 | 3,48 | 2,40-5,04 | < 0,0001 |
| pH>5.8 | pH<5.6 | R.R. | 95%CI | P | ||
| Carbonate-apatite Stones | Total stones | Carbonate Apatite Stones | Total stones | |||
| 99 | 266 | 53 | 295 | 2,07 | 1,55-2,77 | < 0,0001 |
| pH>6.0 | pH<5.9 | R.R. | 95%CI | P | ||
| Struvite Stones | Total stones | Struvite Stones | Total stones | |||
| 20 | 144 | 15 | 358 | 3,31 | 1,75-6.29 | 0,0002 |
Like carbonate apatite phosphate stones, struvite urinary stones form in an alkaline environment. A correlation analysis carried out in seven pH ranges shows a direct dependence of the frequency of detection of struvite stones as the alkaline values of urine increase (Fig. 1, r = 0.889, p = 0.0074). Struvite stones were found with the highest frequency at a urine pH of 7.0-9.0, that is, 3.5 times more often than in patients with a urine pH of 6.1-6.9 (Fig. 1, p < 0.015).
This is due to the fact that crystallization of struvite (MgNH4PO4• 6H2O) is observed at urine pH above 7.0 [34-36], while struvite solubility increases at urine pH below 6.5 [37]. As is known, the main cause of the formation of struvite stones is a urinary tract infection, in which the activity of urease-producing microorganisms (Proteus spp., Klebsiellapneumoniae and Providenciaspp.) leads to increased ammoniagenesis and severe alkalization of urine [35,38]. It should be noted that the increase in the incidence of struvite stones with an increase in urine pH to 7.0-9.0 was not associated with the influence of metabolic risk factors. There was no increase in the excretion of calcium, uric acid, or phosphates and magnesium, which are part of struvite stones. Moreover, the excretion of these substances and ions was significantly lower compared to their maximum values recorded in the urine pH range of 5.6-5.8 (Fig. 1).
This indicates the leading role of urine alkalization in struvite lithogenesis, but not the influence of known metabolic risk factors excreted in the urine on this process. In addition, attention should be paid to the special role of urine pH in regulating the excretion of calcium, uric acid, phosphate and magnesium. The maximum daily excretion of these ions and uric acid is observed at a urine pH of 5.6-5.8 and decreases at a pH above or below these values (Fig. 1). Obviously, the urine pH range of 5.4-6.0 can be considered optimal for the process of excretion of these substances.
The role of urine pH in the regulation of excretion of substances is confirmed by the results of long-term metaphylaxis in patients with removed struvite stones, who were prescribed L-methionine to acidify urine [39]. As a result of the therapy, the urine pH decreased on average from 7.5 to 5.5, while the patients experienced an increase in the excretion of magnesium and uric acid. Calcium excretion showed an increasing trend (p = 0.08). Similar changes in excretion rates with a decrease in alkaline urine pH values were noted previously [17]. In this work, this dependence is presented in Figure 1.
Like carbonate apatite phosphate stones, struvite urinary stones tend to form in alkaline urine. Therefore, with alkaline urine pH values, the relative risk of the formation of such stones is increased compared to acidic pH values. Thus, the risk of stone formation from carbonate apatite at pH > 5.5 is 2 times higher than for urine with pH < 5.6. For struvite stones, the relative risk is 3.3 for urine pH >6.0, compared with urine pH <5.9 (Table 1).
CONCLUSION
As a result of the work, it was shown that the acidity of urine is able to regulate the daily excretion of calcium, uric acid, phosphates and magnesium. Maximum excretion of these metabolic risk factors for KSD occurs at urine pH in the range of 5.6-5.8 and decreases above or below these values. The highest frequency of detection of cases of oxalate urolithiasis is observed at pH 5.6-5.8 and coincides with the maximum calcium excretion, which must be taken into account when carrying out metaphylaxis. At pH values below 5.6-5.8, metaphylaxis of oxalate urolithiasis should be directed to a greater extent at the correction of urine pH disorders, since under these conditions the low level of calciuria does not significantly affect oxalate lithogenesis.
Most often, urate stones form at pH < 5.4-5.5; carbonate apatite – at pH > 5.6-5.8; struvite - at pH = 7.0-9.0, while under these conditions there is a low excretion of metabolic risk factors. It can be assumed that the acidity of urine is more capable of influencing the frequency of formation of urate and phosphate stones than the metabolic risk factors studied in the work, which should be taken into account when carrying out anti-relapse treatment of KSD.
LITERATURE
1.Grases F, Costa-Bauza A, Prieto RM. Renal lithiasis and nutrition. Nutr J 2006;5:23. doi:10.1186/1475-2891-5-23.
2. Barbas C, Garcia A, Saavedra L, Muros M. Urinary analysis of nephrolithiasis markers. J Chromatogr B Analyt Technol Biomed Life Sci 2002;781(1-2):433-55. doi:10.1016/S1570-0232(02)00557-3.
3. Welch AA, Mulligan A, Bingham SA, Khaw KT. Urine pH is an indicator of dietary acid-base load, fruit and vegetables and meat intakes: results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk population study. Br J Nutr 2008;99(6):1335-43. doi:10.1017/S0007114507862350.
4. Thongboonkerd V, Mungdee S, Chiangjong W. Should urine pH be adjusted prior to gel-based proteome analysis? J Proteome Res 2009; 8(6):3206-11. doi:10.1021/pr900127x.
5. Ide H, Kikuchi E, Hagiwara M, Hayakawa N, Hongo H, Miyajima A, et al. Urinary pH levels are strongly associated with bladder recurrence after nephroureterectomy in upper tract urothelial carcinoma patients with a smoking history. Ann Surg Oncol 2016;23(Suppl 5):1029-1038.
6. Cho YH, Lee SY, Jeong DW, Choi EJ, Nam KJ, Kim YJ, et al. The association between a low urine pH and the components of metabolic syndrome in the Korean population: Findings based on the 2010 Korea National health and nutrition examination survey. J Res Med Sci 2014;19(7):599-604.
7. Bihl G, Meyers A. Recurrent renal stone disease-advances in pathogenesis and clinical management. Lancet 2001;358(9282):651-6. doi:10.1016/S0140-6736(01)05782-8.
8. Tiselius HG. A calcium hypothesis of stone formation: an interpretation of stone research during the past decades. Urol Res 2011;39(4):231-43. doi:10.1007/s00240-010-0349-3.
9. Han H, Segal AM, Seifter JL, Dwyer JT. Nutritional Management of Kidney Stones (Nephrolithiasis). Clin Nutr Res 2015;4(3):137-52. doi:10.7762/cnr.2015.4.3.137.
10. Ratkalkar VN, Kleinman JG. Mechanisms of Stone Formation. Clin Rev Bone Miner Metab 2011;9(3-4):187-197. doi:10.1007/s12018-011-9104-8.
11. Worcester EM, Coe FL. Nephrolithiasis. Prim Care 2008;35(2):369-91. doi:10.1016/j.pop.2008.01.005, vii.
12. McKay CP. Renal stone disease. Pediatr Rev 2010;31(5):179-88. doi:10.1542/pir.31-5-179.
13. Grases F, Costa-Bauzá A, Gomila I, Ramis M, García-Raja A, Prieto RM. Urinary pH and renal lithiasis. Urol Res 2012;40(1):41-6. doi:10.1007/s00240-011-0389-3.
14. Wagner CA. Mohebbi N. Urinary pH and stone formation. J Nephrol 2010;23(Suppl 16):S165-9.
15. Türk C, Knoll T, Petřík A, Sarica K, Skolarikos A, Straub M, et al. Guidelines on Urolithiasis - EAU, 2015. URL: https://uroweb.org/wp-content/uploads/22-Urolithiasis_LR_full.pdf.
16. Golovanov SA, Sivkov AV, Drozhzheva VV, Anokhin NV. Metabolic risk factors and urinary stone formation. Study I: Effects of Calciuria and Uricuria. Experimental and Clinical Urology 2017;(1):52 – 57.
17. Golovanov SA, Sivkov AV, Drozhzheva VV, Anokhin NV. Metabolic risk factors and urinary stone formation. Study II: effects of phosphaturia and magnesiumuria. Experimental and Clinical Urology 2017;(2):42 – 48.
18. Rendina D, De Filippo G, De Pascale F, Zampa G, Muscariello R, De Palma D et al. The changing profile of patients with calcium nephrolithiasis and the ascendancy of overweight and obesity: a comparison of two patient series observed 25 years apart. Nephrol Dial Transplant 2013;28 (Suppl 4):iv146-51. doi: 10.1093/ndt/gft076.
19. Cho ST, Jung SI, Myung SC, Kim TH. Correlation of metabolic syndrome with urinary stone composition. Int J Urol 2013;20(2):208-13. doi: 10.1111/j.1442-2042.2012.03131.x.
20. Daudon M, Lacour B, Jungers P. Influence of body size on urinary stone composition in men and women. Urol Res 2006;34(3):193-9. doi:10.1007/s00240-006-0042-8.
21. Manissorn J, Fong-Ngern K, Peerapen P, Thongboonkerd V. Systematic evaluation for the effects of urine pH on calcium oxalate crystallization, crystal-cell adhesion and internalization into renal tubular cells. Sci Rep 2017;7(1):1798. doi:10.1038/s41598-017-01953-4.
22. Parks JH, Worcester EM, Coe FL, Evan AP, Lingeman JE. Clinical implications of abundant calcium phosphate in routinely analyzed kidney stones. Kidney Int 2004;66(2):777-85.
23. Coe FL, Evan AP, Worcester EM, Lingeman JE. Three pathways for human kidney stone formation. Urol Res 2010;38(3):147-60. doi:10.1007/s00240-010-0271-8.
24. Hesse A, Schneider HJ, Berg W, Hienzsch E. Uric acid dihydrate as urinary calculus component. Invest Urol 1975;12(5):405-9.
25. Atsmon A, deVries A, Frank M. Uric Acid Lithiasis. Amsterdam: Elsevier; 1963. pp 423-427
26. Sakhaee K. Epidemiology and clinical pathophysiology of uric acid kidney stones. J Nephrol 2014;27(3):241-5. doi: 10.1007/s40620-013-0034-z.
27. Abou-Elela A. Epidemiology, pathophysiology and management of uric acid urolithiasis: a narrative review. J Adv Res 2017;8(5):513-527. doi: 10.1016/j.jare.2017.04.005.
28. Daudon M, Frochot V. Crystalluria. ClinChem Lab Med 2015;53 (Suppl 2):s1479-87. doi: 10.1515/cclm-2015-0860.
29. Finlayson B, Smith A. Stability of first dissociable proton of uric acid. J Chem Eng Data 1974;19:94-97.
30. Grases F, Villacampa AI, Costa-Bauza A, Sohnel O. Uric acid calculi: types, etiology and mechanisms of formation. Clin Chim Acta 2000;302(1-2):89-104.
31. Grases F, Sanchis P, Isern B, Perello J, Costa-Bauza A. Uric acid as an inducer of calcium oxalate crystal development. Scand J Urol Nephrol 2007;41(1):26-31. doi: 10.1080/00365590600831571/
32. Grases F, Costa-Bauza A, Ramis M, Montesinos V, Conte A. Simple classification of renal calculi closely related to their micromorphology and etiology. Clin Chim Acta 2002\322(1-2):29-36.
33. Grases F, Sanchis P, Perello J, Costa-Bauza A. Role of uric acid in different types of calcium oxalate renal calculi. Int J Urol 2006;13(3):252-6.
34. Elliot JS, Sharp RF, Lewis L. The solubility of struvite in urine. J Urol 1959;81(3):366-8.
35. Hesse A, Heimbach D. Causes of phosphate stone formation and the importance of metaphylaxis by urinary acidification: a review. World J Urol 1999;17:308-315.
36. Siener R, Struwe F, Hesse A. Effect of L-Methionine on the Risk of Phosphate Stone Formation. Urology. 2016;98:39-43. doi: 10.1016/j.urology.2016.08.007
37. Jacobs D, Heimbach D, Hesse A. Chemolysis of struvite stones by acidification of artificial urine. Scand J Urol Nephrol 2001;35:345-349.
38. Hesse A, Tiselius HG, Siener R, Hoppe B. Urinary stones: Diagnosis, Treatment, and Prevention of Recurrence. 3rd ed. Basel: Karger; 2009. 39. Jarrar K, Boedeker RH, Weidner W. Struvite stones: long term follow up under metaphylaxis. Ann Urol (Paris). 1996;30(3):112-7.
| Attached file | Size |
| 1.24 MB |
‹ Ligature stones after kidney transplantation Up Mechanisms of chemopreventive action and effectiveness of the drugs Indigal, Indigalplus and Infemin against prostate cancer ›
Ways to reduce and increase acidity levels
There are medicinal methods to lower or increase acidity levels, as well as recommendations for introducing certain foods into the diet that help normalize pH.
Doctors prescribe intravenous solutions to the patient. They are made on the basis of potassium bicarbonate, as well as products sold in pharmacies for the successful normalization of acidity.
In order to significantly reduce the high acidity of urine, it is recommended to consume foods with low levels of protein. Those foods that have a neutral alkaline load should be consumed.
You also need to eat foods with zero acid formation. These include:
- cucumbers;
- tea;
- ice cream;
- vegetable oil;
- honey.
It is allowed to introduce foods that have negative acidity into food. These are fruits, mushrooms, fresh herbs, fruit juices, and white wine.
The fact is that the division of food according to acidity is quite arbitrary. Each human body is individual and digests food differently. However, you need to gradually adjust the menu in accordance with the recommendations of your doctor.
It is important to remember to normalize your water balance, since people who lead a healthy lifestyle tend to be less likely to suffer from hyperacidity of urine. Water not only normalizes the acidity state in the human body, but also improves the functioning of the renal system
In order to increase acidity, on the contrary, it is necessary to slightly reduce the amount of water consumed, since it significantly increases the level of acidity in the body.
A urine test that determines the Ph level is important because it can give an informative picture of many internal diseases. Therefore, doctors recommend taking a test in a laboratory and monitoring the acidity level at home using test strips.
It is important to study the basic methods of increasing and decreasing acidity and apply them to adjust this indicator. Learn how to use litmus paper to determine acidity from the video:
Learn how to use litmus paper to determine acidity from the video:
Almighty pH: High acidity urine problems for the patient
Professor Henderson and his team carried out the research by culturing E. coli in urine samples with a pH of 6.4-6.5 taken from healthy volunteers. Scientists noticed that the protein siderocalin produced by the body completely killed the colonies of the microbe. The cause of the death of the rods was the aromatic compounds contained in siderocalin and preventing bacteria from absorbing the iron ions they need. The samples showed high activity of macrophages - immune cells that capture and digest pathogenic microbes.
An increase in acidity caused the protein protection to decompose, and the microbes felt at ease. An examination of patients with chronic cystitis revealed that increased acidity of urine aggravated the unpleasant symptoms of cystitis - pain and stinging when urinating, frequent urge to go to the toilet.
Alkalinization gave amazing results. Within 48 hours, the patients' condition improved, and 80% of the patients felt practically healthy. Not only did E. coli disappear from the urine samples, but the number of staphylococci also decreased.
Potassium and magnesium citrates were used as alkalizing drugs, which urologists prescribe in our country.
How to reduce the acidity of urine?
If the acidity of the urine is not normal, the best way to regulate it is to follow a special diet. The patient in this situation will need to consume more foods with zero or negative acid formation.
Your doctor and test strips will help you choose the right products; you will need to use them daily. Regardless of the individual characteristics of the body, patients facing this problem are recommended to consume the following products:
- dairy products;
- bananas, apples, pineapple, oranges, melons;
- fruit juices (natural);

- cucumbers, potatoes, tomatoes, peppers, carrots;
- vegetable oil;
- honey;
- mushrooms;
- coffee;
- beer, white and red wine.
If the patient feels normal when the urine pH deviates, this is not a cause for concern, since the pathology is caused precisely by dietary habits. Alkaline urine does not cause discomfort in the patient, well-being is not tied to the acidity of urine, the norm determines the health of the body. But if, when the indicator changes, a person feels certain ailments, it is necessary to urgently make an appointment with a doctor.
Determination of urine pH
A urine test has three main components:
- Visual inspection.
The doctor or lab technician examines the urine, checking its color, the presence of foreign substances in the urine, such as blood, and seeing if the urine appears foamy. - Test strips for urine analysis
. Involves holding a piece of specially treated or litmus paper in a urine sample. The paper changes color to show how acidic or alkaline the urine is. The paper may change color if other substances such as glucose, white blood cells, bilirubin, or proteins are present in the urine. - Microscopic examination
. A laboratory technician examines a small amount of urine under a microscope to look for particles such as red blood cells, crystals, or white blood cells. They are not usually present in urine and may indicate an underlying medical condition.