Most of us will never be interested in the pH balance of our blood, but proper pH balance is an important aspect of overall health. Many doctors emphasize the importance of reducing acidity and increasing alkalinity in the body through an alkaline diet because a balanced pH level helps protect our body from the inside out. Disease and dysfunction of organs, as doctors say, cannot take root for long in an organism whose acid-base balance is in balance.
What does “pH balance” mean? How do you know when your pH levels are off? The fact is that pH values are related to the balance in the human body between acidity and alkalinity. Your body works hard to maintain a balanced pH every day. In most cases, by eating alkaline foods or following a full alkaline diet, you can help your body defend against harmful microbes and organisms, prevent tissue and organ damage, prevent micronutrient depletion, and protect against immune system dysfunction.
Why is this so? To find out more, you should read this article.
In 2012, the journal Ecology and Health published a review on the health effects of an alkaline diet. The main conclusion from this article was:
“Today, modern people who eat food from current agriculture get much less magnesium and potassium in their diet, as well as significantly less fiber. Their current diet contains too much saturated fat, simple sugars, sodium (salt) and chlorides compared to the diet of their ancestors. This means that such a diet can lead to metabolic acidosis, which does not correspond to our genetic code due to the type of diet." []
The most effective way to maintain a healthy pH balance is to eat plenty of alkalizing plant foods and greatly limit your intake of processed foods .
In addition, there are many different factors that have a serious impact on the acid-base balance: intestinal health , psychological stress, medication, chronic diseases. All of these factors influence how hard the human body must work to maintain normal pH levels.
What is acid-base pH balance? And why is this the key to good health?
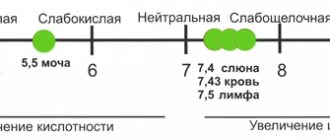
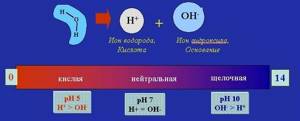
What we call “ pH balance ” is a measure of the activity of hydrogen ions in solutions. [I] pH values are a measure of the acidity or alkalinity of fluids in body tissues. pH values range from 0 to 14. The more acidic the solution, the lower the pH values. More alkaline liquids show higher pH values. The pH scale measures the acidity or alkalinity of many liquids, such as the water in the oceans and seas, not just our blood.
What should the ideal acid-base pH balance be? PH=7 is considered neutral, which means the liquid is equally acidic and alkaline. The pH of the blood serum, as well as the pH of most tissues of our body, should remain around 7.365 , while in the stomach the pH balance is determined to be about 2 units. This strong acidity in the stomach is necessary to process food. Our saliva or urine is also slightly acidic and is in the pH range of 6.4-6.8 in healthy people.
If a person practices an alkaline diet, then it helps him restore the correct level of acid-base balance and helps improve health.
An alkaline diet has been shown to help:
[, , , , ]
- Protection against cardiovascular diseases
- Prevention of calcium accumulation in urine
- Prevention of urolithiasis , kidney disease or damage
- Reduce general inflammation
- Reducing the risk of developing diabetes
- Maintain good bone density
- Reducing the likelihood of muscle cramps
- Protection against vitamin D and related consequences
- Reducing lower back pain
What foods acidify the blood: table
Plan your diet and lifestyle in such a way that you do not have problems with blood alkaline levels. A healthy diet will help you maintain health and youth throughout your life. So, what foods acidify the blood? Table:
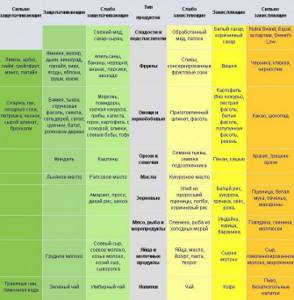
What foods acidify the blood: table
If your blood is highly acidified, change your eating habits. Proper nutrition has been in fashion for several years now, but there are still many people in the world who do not know what this term means.
What causes acid-base imbalance?
Here's the definition of acidosis, a condition where your pH level shifts toward a more acidic state: "...is the overproduction of acid in the blood or excessive loss of bicarbonate from the blood (metabolic acidosis), or the accumulation of carbon dioxide in the blood, which results from poor lung function and depressed breathing (respiratory acidosis).” []
Your body almost always does an excellent job of maintaining your acid-base balance at an optimal level. Unfortunately, you are given the “key” from birth on how hard your body will work to achieve optimal pH values.
Normally, our kidneys maintain proper pH balance and electrolyte levels, including calcium, magnesium, potassium and sodium. But when we are exposed to acidic substances, these electrolytes are used (used up) to combat acidity .
The kidneys begin to excrete more minerals from the body through urine. A highly acidic diet or body health condition causes our body to draw minerals (electrolytes) from our bones, cells, organs and tissues. Our cells have a serious need for sufficient minerals to produce their waste products. Therefore, first of all, with an increase in acidity, a loss of minerals occurs in the bone tissue (bones), which contributes to the development of osteoporosis. When cells work hard in an oxidized environment, the process of accumulation of toxins and pathogens can begin that the cells do not have time to eliminate, and this, in turn, can suppress the immune system.
Once you switch your body to an altered acid-base balance with a predominance of acidity, you force your body to work overtime to keep your blood in the neutral pH zone. This type of strenuous work by the body disrupts the levels of various substances that the body uses to perform its task of maintaining pH. These disorders include decreased potassium levels, imbalanced sodium ratios (our ancestors had a 10:1 potassium to sodium ratio, while modern humans exhibit a 1:3 ratio), decreased magnesium levels , very low fiber intake values, and earlier loss of function. kidney function, especially during aging. [AND]
You cannot cause your blood pH imbalance on your own (which can lead to death), but you can, through your actions, reduce your body's endurance, which will prevent you from experiencing healthy aging . Only helping your body maintain a normal acid-base balance can provide you with healthy years of life.
How to increase acidity and reduce blood pH?
It is bad for the body when the alkaline balance of the blood is greatly increased and has high levels. How to increase acidity and reduce blood pH? Adviсe:
- Eat acid-containing foods - grains, legumes, protein foods (meat, eggs).
- Eat foods rich in dietary fiber.
- Three times a day you can take 1 tablespoon of apple cider vinegar with honey.
- Vitamin C lowers pH levels.
- Do breathing exercises with deep breaths.
- If there are no medical contraindications, you can use dietary supplements - food enzymes and others.
- Correct the vitamin status in the body by taking multivitamin complexes.
Also, to increase acidity, it is necessary to carry out prevention and adequate treatment of the genitourinary system.
Types of acidosis
There are five main types of what doctors call "metabolic acidosis." This condition means that the body has a poor acid-base pH balance or is working too hard to maintain a healthy pH.
Diabetic ketoacidosis is sometimes mistakenly confused with a state of ketosis. Diabetic ketoacidosis occurs when a person with diabetes's body cannot cope with changes in its condition and the liver produces dangerously high amounts of ketone bodies. This condition is usually diagnosed when blood sugar levels exceed 13 mmol/L.
Hyperchloremic acidosis condition with excessive vomiting or diarrhea. With this form of acidosis, there is a decrease in the level of sodium bicarbonate and an increase in the concentration of chloride in the blood plasma.
Lactic acidosis – Too much lactic acid can lead to acidosis. According to scientific journals, “causes of this condition may include chronic alcohol use (alcoholism), cardiac arrest, cancer, liver failure, decreased oxygen levels in the air, and low blood sugar.” Additionally, prolonged exercise can lead to a buildup of lactic acid in the blood.
Renal tubular acidosis - If your kidneys cannot produce enough acid in your urine, your blood may become acidic.
Dietary acidosis is a recently recognized form of acidosis. Dietary acidosis (or “diet-induced acidosis”) is the consequence of eating highly acidic (not lemon) foods, which places a very high burden on the body, increasing the risk of various diseases and deteriorating overall functioning. body. []
Symptoms of changes in blood pH
If the blood pH is outside the healthy range, he may begin to experience certain symptoms.
The symptoms people experience will depend on whether their blood becomes more acidic or less acidic.
Some symptoms of acidosis include:
- headache
- confusion
- fatigue
- lethargy and drowsiness
- cough and shortness of breath
- uneven or rapid heartbeat
- upset stomach or nausea
- muscle cramps or weakness
- loss of consciousness and coma
Symptoms of alkalosis include:
- confusion and confusion
- trembling hands
- numbness or tingling in the legs, arms, or face
- muscle cramps
- vomiting or nausea
- coma
The best ways to maintain the correct acid-base pH balance
First, you can reduce your risk of falling out of healthy pH by looking at how your lifestyle and habits may be affecting your nutrient levels, gut function, and immune system.
Below are the main factors that contribute to the growth of acidity (acidosis) in your body
- Alcohol and drug use (including acetazolamide, opioids, tranquilizers, and aspirin)
- Excessive use of antibiotics
- Kidney disease or kidney dysfunction
- Poor digestion and poor gut health
- Eating a lot of processed and refined foods containing salt, preservatives, etc. []
- Low dietary intake of potassium, calcium and other beneficial minerals []
- High consumption of artificial sweeteners, food colors and preservatives
- Pesticides and herbicides that may remain in plant products
- Chronic psychological stress
- Sleep disorders, such as apnea
- Decreased nutritional levels in food due to industrial agriculture and poor topsoil quality
- Low levels of fiber in the diet
- Lack of exercise (sedentary lifestyle)
- Excess animal meat in the diet
- Excessive ingestion of cosmetics and plastic residues into the body
- Exposure to chemicals from household cleaning products, building materials, radiation from computers, cell phones and microwave ovens
- Environmental pollution
- Poor chewing and eating habits (eating quickly without chewing thoroughly)
- Lung disease or damage, including emphysema, chronic bronchitis, severe pneumonia, pulmonary edema, and asthma
Alkalinity level of exhaled air condensate
Exhaled air condensate (EBC) is exhaled air condensed due to cooling (up to 4 ° C or at negative temperatures). EBC shows changes in exhaled fluid. This method of testing or diagnosing lung disease and other symptoms is not approved. The exhaled air was used to study the lungs by the Russians.
EBC analysis is a developing method for studying respiratory tract pathology. The influence of food and drink on the composition of breath condensate has not been systematically studied. The measure of acidity is determined using a blood gas analyzer. Drinking Coca-Cola causes changes in the pH of the blood and urine. Drinking influences the composition of the condensate and contributes to the variability of the pH of the condensate.
The alkalinity level of air condensate is also determined using a special pH meter. The average pH value of condensate in healthy people is from 7.8 to 8.1. pH values below 7.4 are considered abnormal. With exacerbation of diseases, the hydrogenation potential decreased. After treatment of pneumonia, the pH increased. Drops from the stomach, saliva and exhaust gases are added to the condensate. This affects the concentration of hydrogen ions.
There are several ways to collect condensation from exhaled air. Collect condensation as liquid, not ice. Rapid freezing of air vapor during exhalation removes volatile substances from solution, which contribute to increasing the hydrogenation potential, and affects the results.
KVV components:
- aerosol particles from the respiratory tract
- drops of water vapor around the aerosol
- water-soluble gases.
The content of EBC is influenced by physical activity, nutrition, pregnancy and other factors. Collection of EBC and analysis of alkalinity measures is simple, non-invasive, and inexpensive. The method is suitable for non-invasive analysis during long-term follow-up of individual patients, which is provided by a portable device for collecting EBC at home. Today, ECV is a way to assess the role of airway acidification in respiratory diseases.
How can you help your body achieve a neutral pH level?
Below are steps to help you maintain a better acid-base pH balance.
Reduce intake of acidic foods
If you currently eat a " standard Western diet " then you may need to change your diet to be more alkaline. Here is a list of acidic foods that should be limited in your diet or completely excluded from your diet:
- Store-bought processed meats, cold cuts, hot dogs, sausages, salami.
- Foods High in Salt
- Sugar and sugar products
- Processed cereal grains such as corn, wheat, barley, sorghum, millet and rye (including flour from these cereals)
- Regular meat (beef, chicken and pork)
- Fried foods
- Milk and dairy products
- High glycemic index foods including white rice, white bread, pasta, breakfast cereals, etc.
- Caffeine
- Alcohol

There are some "sour" foods that contain many essential nutrients, so rather than being completely eliminated from your diet, they can be consumed in moderation as part of a healthy diet.
- Most high protein foods such as meat and eggs
- Lentils and other legumes
- Oats
- Brown rice
- Whole wheat bread
- Walnut
Go on an alkaline diet
If you plan to follow an alkaline diet to balance your pH, then your diet should be rich in green plants and other alkaline foods. It is also wise to buy more organically grown food (not from standard farming methods, but from farms or private gardens). These foods are grown in a more organic environment, in soil that is high in minerals, which tend to be more alkalizing and contain more vitamins.

HERE IS A LIST OF FOODS THAT WILL CONTRIBUTE TO AN ALKALINE DIET
- Leafy green vegetables - kale, chard, beet greens, dandelion greens, spinach, wheatgrass, alfalfa, etc.
- Other non-starchy vegetables - mushrooms, tomatoes, avocados, radishes, cucumber, broccoli, oregano, garlic, ginger, green beans, endive, cabbage, celery, zucchini and asparagus
- Raw Foods – Raw fruits and vegetables are biogenic or “life-giving” foods for our body. Cooking, especially cooking, can reduce the content of alkali minerals. Increase the amount of raw food in your diet and try lightly steamed vegetables. Ideally, try to consume most of your foods raw, or only lightly cooked (such as steamed).
- Superfoods (healthy foods) - maca root, spirulina, sea vegetables, bone broth and dried greens powder containing chlorophyll
- Healthy fats: Coconut oil, olive oil, fish oil, fat from farmed or home-raised animals (these foods can be good additions to your diet, even if they are not necessarily alkalizing).
- Starchy plants include sweet potatoes, turnips and beets.
- Plant proteins - almonds, beans, kidney beans and most other legumes
- Most fruits - Surprisingly, sour-tasting fruits such as lemon or grapefruit do not create acidity in the body. They do just the opposite and contribute to alkalization of the body. Citrus fruits, dates and raisins are highly alkalizing and can help prevent acidosis. []
- Vegetable juices (green drinks) are drinks made from green vegetables and herbs in powder form. Such foods are very rich in chlorophyll . Chlorophyll is structurally similar to our own blood and alkalinizes the blood. []
- Apple cider vinegar has a sour taste, but is able to restore the acid-base pH balance well.
Depending on your current health status and your health goals, you could achieve better acidity status by following an alkaline, very low-carb, ketogenic diet. The keto diet (ketogenic) also maintains pH balance in the body by eating healthy fats, green leafy vegetables, vegetable juices and superfoods (healthy foods). But it’s worth knowing all the pros and cons of the ketogenic diet before you start practicing it.
Most high-protein foods are acid-forming, so if you eat a lot of meat and animal products, it's important to balance out their acidic influences with alkalizing plant-based foods. [] If you are following a low-carb diet to reduce acidity, then in addition to the foods mentioned above, you can add beans, nuts and some small amounts of starchy foods (they contain a lot of carbohydrates and sugar).
Drink alkaline water
According to the US Water Research Center, “...the normal pH range for surface water systems is 6.5 to 8.5 and for groundwater systems is 6 to 8.5...” [] This means that there are many varieties of water with different pH.
When water has a pH level of less than or around 6.5, it can be characterized as “acidic and corrosive.” Such water can leach metal ions such as iron, manganese, copper, lead and zinc from aquifers, faucets and pipes, and may also contain toxic metals and have a sour taste. The best way to change the problem of acidic water (low pH) is to use a special neutralizer that can increase the pH .
Water that shows a pH between 9 and 11 can be considered alkaline. Distilled water has a neutral pH of about 7. [] Adding baking soda to acidic water can also increase the alkalinity of the water.
Water filtered with a reverse osmosis filter is slightly acidic, with a pH of just under 7. Distilled water and filtered water may not become too alkaline, but if you are not too concerned about the acidity of such water, then such waters can be considered a better option in comparison. with tap water or plastic bottled water, which is more acidic.
Reduce the entry of drugs, toxins and chemicals into your body
Many different medications, chemicals and toxins can disrupt the acid-base pH balance and contribute to increased acidity in the body. Such products include: alcohol, caffeine, acetazolamide, opioids, sedatives, carbonic anhydrase inhibitors, nonsteroidal anti-inflammatory drugs (NSAIDs), and aspirin . []
It is important to exclude as much as possible from your life all influences that can lead to the constant use of various medications . For example, lack of sleep, psychological stress, sedentary lifestyle and even allergies can cause problems with your health, which will push you to take various medications.
Try to determine what steps you can take to naturally reduce your need for medications. If you live or work in an environment with a lot of air pollution , then it is worth taking steps to protect yourself as much as possible from such pollution.
Where can I get a blood PH test?
Laboratory tests are much more accurate than those obtained at home. If you decide to take a blood PH test in a special laboratory, you can go to the clinic at your place of registration or to any private clinic. The analysis will be ready on the day of blood sampling. The doctor himself may suggest that you take a test during a routine examination or preventive procedures if there are deviations in your health.
Testing the pH level of acid-base balance
This is how you can check your own pH level
- You can test your pH by purchasing pH test strips from your local health food store or pharmacy.
- pH measurements can be made using saliva or urine. The second morning urination gives the best pH results in terms of accuracy.
- You compare the colors on the test strip to the pH scale chart that comes with this test strip kit.
- During the day, the best time to check your pH is one hour before meals and two hours after meals.
- If you are testing your saliva, the ideal pH range for health is between 6.8 and 7.3 (remember, optimal pH is around 7.365).
Source:
Code of Life
We recommend:
An integrated approach to cancer treatment
How to measure blood PH at home with a device, test strips?
Of course, if you have any health problems, you should go to the clinic to see a doctor. But it often happens that we don’t have time to go to the hospital - don’t be upset. You can measure blood PH at home with a device or test strips.
A special device is sold at a pharmacy or any medical equipment store. It is inexpensive, but very useful for measuring blood PH at home. If you can’t find such a device, use test strips. They are sold in every pharmacy and cost pennies. If you don’t find any strips or a tester at the pharmacy, you can order everything you need online.
How to measure blood PH at home with test strips - tips:
- Prick the finger of your right hand with a scarifier, which is also sold in pharmacies.
- Squeeze some blood into a small container. It's good if you have a laboratory test tube.
- Dip the test strip into this blood, hold it for a few seconds, remove it from the tube, and evaluate the result.
- The scale for determining the alkaline reaction in the body is on the package with strips. Compare the color and find out the result.
When measuring PH values using the device, it is faster, easier and more convenient. You do not need to pierce your finger, the device will do everything on its own: puncture, sampling and give the result.
Blood buffer systems
Products of an acidic or basic nature constantly enter the human blood, but for some reason nothing happens? It turns out that everything is provided for in the body; buffer systems are “on duty” around the clock to guard the constancy of pH, which resist any changes and do not allow the acid-base balance to shift in a dangerous direction. So, in order:
- The bicarbonate system opens the list of buffer systems; it is also called the hydrocarbonate system. It is considered the most powerful, since it takes on a little more than 50% of all blood buffering abilities;
- The second place is taken by the hemoglobin buffer system, it provides 35% of the total buffer capacity;
- The third place belongs to the buffer system of blood proteins - up to 10%;
- In fourth position is the phosphate system, which accounts for about 6% of all buffering capabilities.
These buffer systems, in maintaining a constant pH, are the first to resist a possible shift in the pH value in one direction or another, because the processes that support the vital activity of the body are ongoing, and at the same time, products of either an acidic or basic nature are constantly released into the blood. Meanwhile, for some reason the buffer capacity is not depleted. This happens because the excretory system (lungs, kidneys) comes to the rescue, which reflexively turns on whenever there is a need - it removes all the accumulated metabolites.