The skin is the largest specialized human organ, with an area of 2 m2 and a mass of almost 3 kg. It performs a number of important functions. In particular, the skin is a barrier organ and, most importantly, like the thymus, it is the site where certain types of immune cells mature and immunological reactions occur. In principle, the skin barrier contains all types of cells capable of carrying out a wide range of immune reactions. This gives grounds to consider the skin an organ of the immune system.
In the early 80s. In the 20th century, the concept of skin lymphoid tissue—skin-associated lymphoid tissue (SALT)—was formulated, which continues to develop today. In accordance with modern views, along with lymphocytes, the immune system of the skin should include neutrophils, mast cells and eosinophils, Langerhans cells and keratinocytes [3, 4, 20].
Lymphocytes
Lymphoid cells are characterized by recycling - constant exchange between blood, lymph and organs containing lymphoid tissue. Another feature of this cell population is homing—the colonization of certain areas of lymphoid organs and tissues. Therefore, intradermal lymphocytes differ from those circulating in the peripheral blood. To study the population composition of skin lymphocytes, methods of immunohistochemistry and “skin window” were used (determining the percentage of cells in a print from a small area of skin after removing the surface layer of the epidermis). This made it possible to establish that normally skin lymphoid cells are predominantly T-lymphocytes: CD5+ - 19%, CD3+ - 48%, CD25+ - 26%, CD4+ - 33%, CD22+ - 18% [7, 14]. They all have a fairly specific common marker - cutaneous lymphocyte antigen (CLA), which is considered a receptor that controls the affinity of T cells for the skin. CLA is a membrane adhesive molecule that mediates T-lymphocyte binding to the endothelium of postcapillary venules of the skin and its passage into the dermis. CLA-positive T cells account for 10–15% of circulating blood cells. The population of CLA-positive T cells is represented by several subpopulations that differ in receptor status and functional activity [10, 11, 12]. All CLA-positive T cells are characterized by the expression of cutaneous T-cell chemoattractant (CTACK), which “attracts” T-lymphocytes from the circulation into the skin, primarily during various inflammatory processes. The totality of clinical and experimental data accumulated today shows that CTACK plays an important role in the immune response of the skin. Its most significant pathogenetic role is as a pro-inflammatory factor in diseases such as atopic and contact dermatitis [13].
In addition, most T-lymphocytes of normal skin of a healthy person have receptors for other chemokines - biologically active substances that control the migration of cells, in particular lymphocytes. This contributes to their active participation in various immunological reactions, both physiological and pathological [1, 6, 21].
Skin T cells are capable of differentiating into cytotoxic or memory cells (CD45RO). Memory cells also express cutaneous lymphocyte antigen (CLA), are formed in the lymph nodes that drain the skin, and return to the skin during inflammation. Normally, they participate in the formation of immunity in the skin, and in pathology they take part in the pathogenesis of cutaneous T-cell lymphoma, transplant rejection, atopic dermatitis, etc. [2, 5, 10, 17]. About a third of skin lymphocytes are T helper cells (CD4+). In recent years, it has been shown that this subpopulation of cells is represented by two varieties—Th1 and Th2, which differ primarily in the spectrum of cytokines produced. Normally, there is a certain balance between these cells; In skin diseases, the Th1/Th2 ratio changes. For example, during inflammatory processes, the activity of Th1 lymphocytes increases [1, 8, 12, 15]. Thus, skin lymphocytes represent a heterogeneous cell population, in which cells of the recirculating pool and specific skin lymphocytes are present. The latter are characterized by a unique set of cellular receptors that determine their affinity for the skin, as well as a certain set of cytokines produced, allowing them to participate in various cellular reactions that ensure skin repair.
Neutrophils
Neutrophils are found in normal skin in small quantities, but during acute inflammatory processes their number increases significantly. In addition, neutrophil granulocytes participate in the regulation of reparative processes by interacting with other cells (macrophages, keratinocytes). One of the mechanisms of this interaction is the production of neutrophilokines, which stimulate the secretion of growth factors by fibroblasts and lymphocytes, which in turn induce the proliferative activity of regenerating tissue cells [3, 18].
Interpretation of results
An increase in RET is called reticulocytosis. In adults, it may indicate significant recent or ongoing bleeding, anemia, hemolysis, and other pathologies. Reduced levels indicate that the normal process of formation of new red blood cells is disrupted.
In children, normal values vary significantly depending on age. For infants, high levels are normal; they decrease with age. During adolescence, normal values differ significantly depending on gender.
Mast cells and eosinophils
Mast cells (MCs) and eosinophils of the skin are involved in various pathological processes, primarily in allergic ones. When an allergen penetrates the skin, it interacts with eosinophils and mast cells that carry IgE antibodies on their surface. As a result of this interaction, cell activation and degranulation occurs with the subsequent release of various mediators (substance P, interleukins 1 and 6, chemokines). They promote the migration of other immunocompetent cells to the site of the pathological process and support the activity of the inflammatory reaction. The number and functional activity of these cells change differently in various skin diseases. In addition, MCs and eosinophils play a role in the pathogenic effects of stress on the skin [2, 6, 9].
Langerhans cells
Langerhans cells (CL) are specialized cells of the epidermis and make up 2-3% of the total number of its cells.
They are one of the forms of dendritic cells that are of monocyte-macrophage origin and perform the most important immune functions in the body, primarily as antigen-presenting cells. Dendritic cells are a key link connecting acquired and innate immunity [16]. During inflammation and other processes associated with antigenic stimulation, CL acquire motor activity, leave the epidermis with a flow of tissue fluid and, moving through the lymph, undergo certain morphological transformations, as a result of which they become so-called “veil” cells. Reaching the lymph nodes, they actively interact with other immunocompetent cells and present antigens to them. CLs are able to interact with various types of T cells, thus modulating various types of immune reactions (inflammation, autoimmunity). In addition, CLs are directly involved in the destruction of bacteria in the skin.
Interpretation and normal values of the main blood test parameters
| How is it designated? | What does it mean | Norm for women | Norm for men |
| R.B.C. | Red blood cells | 3,5-4,5 | 4,0-5,5 |
| WBC | Leukocytes | 4-9 | |
| PLT | Platelets | 180-320 | |
| HGB | Hemoglobin | 120-140 | 130-170 |
| MCV | Average erythrocyte volume | 82-98 | 81-95 |
| MCH | Average HGB level in erythrocyte | 26-32 | |
| MCHC | Average concentration of red blood cells in HGB (%) | 31-38 | |
| HCT | Hematocrit (in%) | 35-44 | 40-50 |
| RET | Reticulocytes (%) | 0,2-1 | |
| ESR | ESR (mm/h) | 2-15 | 1-10 |
| CPU | Color | 0,85-1,05 | |
Cytokines - bioregulators of immune reactions
Recent decades have been characterized by rapid accumulation of data on a new class of immunoregulatory molecules—cytokines. They include a huge number of different substances, including interleukins, which perform a communicative function between immunocytes and have various regulatory effects both within the immune system and in other organs and tissues. Currently, most of the known interleukins have been found in the skin: their functions are associated with the skin, and disruption of production underlies the pathogenesis of a number of skin diseases, in particular psoriasis and atopic dermatitis [2, 6, 7].
What indicators does the blood test contain?
Donating blood for testing is necessary during planned hospitalization, to assess the effectiveness of the therapy, and during pregnancy. To make an accurate diagnosis and prescribe treatment, the doctor always prescribes a general blood test. Material for research is taken from a finger or from a vein. The second option is preferable, since venous blood more accurately shows the level of hemoglobin and red blood cells.
First of all, red blood cells, white blood cells and platelets are analyzed, as well as:
- Hemoglobin level.
- Erythrocyte indices.
- Hematocrit level.
- Reticulocyte count.
Additionally, the erythrocyte sedimentation rate (ESR), color and blood clotting period are determined.
An extended study involves indicating the leukocyte formula, including the count of eosinophils, lymphocytes, monocytes, band and segmented neutrophils.
The immune system of the skin during infectious and non-infectious lesions
The skin immune system is involved in the implementation of both innate and acquired immunity. Its role is most significant when the integrity of the barrier is disrupted and microorganisms penetrate the dermis. In this case, SALT reacts as a single functional system. In antigen-presenting cells, antigen processing and presentation occur, during which CLs turn into dendritic cells and move through the dermis to the lymph nodes. As a result, they acquire the ability to interact with T helper cells, which then activate B cells and partially differentiate into effector lymphocytes and memory cells. Memory T cells carrying CLA are able to migrate from the bloodstream into the epidermis; They are the ones that predominate in the skin. As a result of an increase in the number of T cells in contact with the most “relevant” antigens, an amendment is made to the antigen recognition repertoire of T lymphocytes. This determines the activity of the immune response.
In non-infectious skin lesions, such as trauma, the immune system is actively involved in the healing of the skin wound. Skin wound healing is a dynamic, interactive process involving mediators, blood cells, extracellular matrix, and mesenchymal cells, which consists of three phases: inflammation, granulation tissue formation, and tissue remodeling. Inflammation is a reaction of the body in general and the skin in particular to injury. The leading role in its development belongs to blood cells - neutrophils. They not only participate in hemostasis, but also secrete biologically active substances.
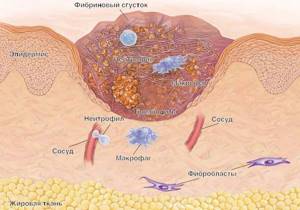
As a result, monocyte-macrophages are activated, which serve as a link between inflammation and regeneration. Activation of these cells leads to the induction of epidermal proliferation. It should be noted that re-epithelialization begins within a few hours after the injury. Initially, it occurs due to the reduction of intracellular tonofilaments, which increases the migratory ability of epidermal cells. After about four days, a newly formed stroma (granulation tissue) is detected in the wound. Under the influence of various cytokines produced by immunocompetent cells, fibroblast differentiation, collagen synthesis, and new vascular formation occur in it. Cytokines, including growth factors (epidermal, transforming, platelet, endothelial and others), take an active part in these processes. Collagen metabolism, the appearance of myofibroblasts in granulation tissue, proliferation of keratinocytes and a number of other cellular events that complete the “maturation” of granulation tissue lead to the formation of a skin scar, which indicates restoration of tissue integrity and completion of the reparative process [19, 21].
Thus, all types of immune response are represented in the skin - innate and acquired (adoptive), cellular and humoral. Thanks to this, both a nonspecific protective function (immunoglobulins, lysozyme, lactoferrin, defensins, phagocytosis) and primary recognition of the antigen with its subsequent presentation and proliferation of antigen-specific T cells are possible. As a result, both cytotoxic reactions and antibody formation occur in the dermis. It must be emphasized that a feature of the skin as an immune organ is the relative predominance of innate immunity over acquired immunity, and in the innate immune system of the skin, cellular factors, in turn, prevail. Analysis of numerous scientific data suggests that immune reactions are related to most physiological and pathological processes occurring in the skin.
Rh factor inheritance
According to Dmitry Okunev, blood belonging to each of the groups (ABO system) and the presence of Rhesus is considered genetically determined and unchangeable. Inheritance of the Rh factor is encoded independently of groups by three pairs of genes. The genotype of a Rh-positive person is homozygous - DD or heterozygous - Dd. The genotype of a Rh negative person is dd.
— Even while the baby is in the maternity hospital, in 1% of cases hemolytic disease of the newborn occurs - a terrible disease accompanied by the death of red blood cells. The pathogenesis of hemolytic disease develops when the blood of mother and fetus is incompatible according to the Rh factor and the ABO system, says Izvestia’s interlocutor.
Blood for blood
Photo: TASS/Egor Aleev
Early diagnosis of hemolytic disease of newborns occurs in the prenatal period. At the same time, doctors exclude an immune conflict between mother and fetus by determining the blood type and Rh factor of the mother and father. If Rh is negative, doctors find out about early pregnancies, abortions, and blood transfusions. During pregnancy, the presence of anti-Rhesus antibodies is determined three times and an ultrasound is performed.

Prison instead of a Nobel Prize: a scientist who saved children from HIV broke their genes
Chinese geneticist who changed the genes of IVF children will spend three years behind bars
SALT function disorders
Extensive experimental and clinical material has shown that dysfunction of SALT—T-cell reactivity, cytokine production, chemokine expression on cells, intercellular interactions, and other immunological reactions—lead to the development of a number of diseases, any of which is accompanied by changes in the appearance of the skin. These can be inflammatory skin diseases (boils, acne), atopic dermatitis, psoriasis, T-cell cutaneous lymphoma [5, 16, 17]. It is known that age-related changes in the skin are also associated with changes in its immunological functions. In aging skin, mononuclear infiltration, a decrease in the number of Langerhans cells and changes in the production of cytokines by immunocompetent cells that affect the proliferation and differentiation of skin cells are observed.
The variety of cells that make up the immune system of the skin, as well as the variety of their functions, explain the fact that at the skin level all types of immunopathological syndromes (immunodeficiency, autoimmune, allergic, lymphoproliferative) can manifest. Immunodeficiency syndrome is manifested, for example, by furunculosis and other purulent-inflammatory processes. With defects in phagocytosis, the skin becomes susceptible to many bacterial and fungal infections, but the immune response to any antigen is impaired because antigen presentation is affected.
Allergic (hyperergic) syndrome occurs quite often and occurs in contact and atopic dermatitis. The phenomena of hyperergy are also characteristic of psoriasis. Autoimmune syndrome also has skin manifestations (scleroderma, systemic lupus erythematosus). An example of a lymphoproliferative syndrome is T-cell lymphoma of the skin (mycosis fungoides).
Diagnosis of all these conditions is based on clinical signs. For example, for an immunodeficiency disease, these will be criteria such as a recurrent course of an infectious skin lesion, its protracted course despite adequate pharmacotherapy, a tendency towards generalization of the infectious-inflammatory process in the skin, resistance to antimicrobial therapy, the predominance of necrotic changes over inflammatory ones in the lesion, discrepancy between local and systemic manifestations of skin infection. There are no specific tests characterizing the state of skin immunity in practical medicine. A dermatologist can rely on standard immunological blood parameters. In scientific studies, morphological (histological) assessment of immunocompetent skin structures, the “skin window” method and some others are used.
How to improve skin immunity?
Pathology of the immune system leads to the development of immune-dependent pathology. Therefore, the need to stimulate skin immunity when it is suppressed is pathogenetically justified. For these purposes, drugs such as Polyoxidonium and Likopid may be recommended. Some immunomodulators (for example, Riboxin) can be used both for systemic and local use, including in mesotherapy techniques. In this case, intradermal injections primarily affect the immune system of the skin, and systemic use leads to the activation of lymphopoiesis in the thymus and lymph nodes. In other words, the choice of method of drug administration (local or systemic) should be based on the nature of immune disorders - both in the skin and in the body as a whole.
Nonspecific adaptogens (vitamin-microelement complexes, aralia tincture, etc.) also have a moderate immunotropic effect. We discovered immunoactive properties in organic silicon, which is widely used in mesotherapy practice. In the treatment of diseases caused by increased reactivity of the immune system (psoriasis, lymphoma), immunosuppressants (cyclosporine) are used. The latest achievement in immunopharmacology is the use of monoclonal (highly specific) antibodies as inhibitors of the immune system.
When improving the immune status of the skin, it should be remembered that the skin immune system, morphologically represented by SALT, on the one hand, is a fairly autonomous department of the body’s immune system, and on the other, has close morphofunctional and regulatory relationships with it. Disturbances in normal immune reactions in the skin lead to the development of many dermatological diseases and the vast majority of aesthetic problems, including premature aging of the skin. It is not surprising that the skin is a target for immunotherapeutic interventions, in particular immunomesotherapy. We plan to consider this issue in more detail in future publications.
Literature
- Belova O. V., Arion V. Ya., Sergienko V. I. The role of cytokines in the immunological function of the skin. Immunopathology, allergology, infectology 2008; No. 1:41-55.
- Borovik T. E., Makarova S. G., Darchia S. N., Gamaleeva A. V., Gribakin S. G. Skin as an organ-immune system. Pediatrics 2010;№2:10-18.
- Dolgushin I. I., Bukharin O. V. Neutrophils and homeostasis. Ekaterinburg: Ural Branch of the Russian Academy of Sciences, 2001.
- Kashutin S. L., Dobrodeeva L. K. Content of immunocompetent cells in the skin of practically healthy people. Honey. immunology 2000; 2(No. 2):128–129.
- Kokhan M. M., Kuklin I. A., Bazarny V. V. Atopic dermatitis and malignant lymphomas of the skin. Allergology and Immunology 2000; 1(No. 2):72.
- Yarilin A. A. Skin and immune system. Cosmetics and medicine 2001; No. 2:5-13.
- Aguilar A. Skin associated lymphoid tissues (SALT). Its normal and pathological function. An R Acad Nac Med 2006; 123:367–377.
- Albanesi C., Scarponi C., Sebastiani S., Cavani A. A cytokine-to-chemokine axis between T-lymphocytes and keratinocytes can favor Th1 cell accumulation in chronic inflammatory skin diseases. J Leukocyte Biol 2001; 70:617–623.
- Babina M., Guhl S., Stärke A., Kirchhof L. Comparative cytokine profile of human skin mast cells from two compartments—strong resemblance with monocytes at baseline but induction of IL-5 by IL-4 priming. J Leukocyte Biol 2004; 75:244–252.
- Clark RA, Chong B., Mirchandani N. The vast majority of CLA+ T cells are resident in normal skin. J Immunology 2006; 176:4431–4439.
- Fuhlbrigge RC, Kieffer JD, Armerding D., Kupper TS Cutaneous lymphocyte antigen is a specialized form of PSGL_1 expressed on skin-homing T cells. Nature 1997; 389: 978–981.
- Hudak S., Hagen M., Ying L., Daniel C., Oldham E., McEvoy LM, Bowman EP Immune surveillance and effector functions of CCR10+ skin homing T cells. J Immunol 2002; 169:1189–1196.
- Kagami S., Sugaya M., Minatani Y., Ohmatsu H. Elevated serum CTACK/CCL27 levels in CTCL. J Invest Dermatol 2006; 126:1189–1191.
- Kanitakis J. Immunohistochemistry of normal human skin. Eur J Dermatol 1998; 8:539–547.
- Lewis JM, Girardi M, Roberts SJ, Barbee SD, Hayday AC Selection of the cutaneous intraepithelial gammadelta+ T cell repertoire by a thymic stromal determinant. Nat Immunol 2006;8:843–850.
- Lipscomb MF, Masten BJ Dendritic cells: immune regulators in health and disease. Physiol Rev 2002; 82:97–130.
- Robert C., Kupper TS Inflammatory skin diseases, T cells, and immune surveillance. N Engl J Med 1999; 341:1817–1828.
- Schaerli P., Britschgi M., Keller M. Characterization of human T cells that regulate neutrophilic skin inflammation. J Immunol 2004; 173:2151–2158.
- Singer AJ, Clark R. Cutaneous wound healing. N Engl J Med 1999; 341:738–746.
- Streilein JW Skin_associated lymphoid tissue. Immunol Ser 1989; 46:73-96.
- Werner S., Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev 2003; 83:835–870.