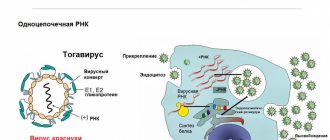
Toxoplasma gondii is a coccidia that is the most common intracellular parasite of warm-blooded animals.
Infection of warm-blooded mammals occurs by ingestion of any of the three stages of T. gondii (sporozoite, tachyzoite, bradyzoite) and transplacentally. The pathogen can be transmitted to kittens through the milk of an infected mother.
Completion of the sexual phase of the Toxoplasma life cycle occurs only in the gastrointestinal tract of cats (enteroepithelial cycle). Upon completion, cysts that are resistant to environmental factors are released in the feces. 1-3 days after contact with oxygen and at favorable temperature and humidity, sporozoites develop in the cysts, this process is called sporulation. After ingestion of oocysts in the small intestine of cats or intermediate hosts of the parasite, they emerge as sporozoites. During the period of active infection, as a result of the proliferation of sporozoites in epithelial tissues, tachyzoites are formed, which migrate into the blood and lymph.
T. gondii can penetrate most mammalian cells, in which the parasite reproduces asexually until cell destruction. In the presence of the correct immune response, tachyzoite replication slows down, and slowly dividing bradyzoites appear, which are located inside cysts in extraintestinal tissues. These tissue cysts can quickly form in the central nervous system, muscle tissue and internal organs. Bradyzoites remain viable in tissue cysts throughout the life of the host. Seroprevalence to T. gondii varies depending on the animal's lifestyle; in general, there is a direct correlation between seroprevalence and age, which is associated with an increased risk of contact with the pathogen. It is also noted that this indicator depends on the possibility of animals having access outside the premises, where they can come into contact with infested intermediate hosts.
The development of clinical toxoplasmosis depends on the characteristics of the pathogen and the host’s immune response. Some strains have a higher pathogenicity, others differ in their affinity for certain types of tissue, such as eye pathologies in cats with toxoplasmosis. If the immune response is insufficiently effective after primary invasion, extremely intense replication of tachyzoites occurs, causing tissue necrosis, which is the main cause of the observed pathological disorders. Diseases accompanied by immunosuppression, such as FIV infection, can cause activation of toxoplasmosis. The vast majority of cats infected with T. gondii never develop clinical symptoms. In dogs, the infection manifests itself as disorders of the respiratory, neuromuscular systems and gastrointestinal tract. The mild form of the disease is often asymptomatic. Generalized toxoplasmosis most often occurs in dogs with immunosuppression, such as canine distemper, or those receiving treatment with cyclosporines to prevent transplant rejection.
Diagnosis of toxoplasmosis should be carried out comprehensively, taking into account clinical signs and the results of serological and other types of studies. The IgM titer will increase in the first two weeks after infection of the animal and can persist for up to 12-15 weeks from the moment of infection. An increase in the IgM antibody titer without an increase in the IgG titer indicates the phase of active infection. The production of IgG class antibodies begins in the third week from the onset of the animal’s illness. This class of immunoglobulins can persist and perform a protective function for several years, and sometimes for life. Therefore, if infection is suspected, it is important to examine paired sera taken several weeks apart. An increase in antibody titer in a repeat test will confirm the presence of the disease. IgG (but not IgM) can be transmitted to fetuses from the mother. The detection of high titers of class IGg antibodies to Toxoplasma gondii in newborn animals may indicate infection of the mother during pregnancy and transplacental transmission of infection.
Complexes with this research
Pregnancy planning.
Diagnosis of infections 14 tests for expectant mothers 8,620 RUR Composition Miscarriage Identification of the main causes of miscarriage 40,070 RUR Composition
IVF planning Examination for preliminary preparation of a woman for the IVF procedure RUR 12,990 Composition
IN OTHER COMPLEXES
- TORCH infections. Avidity of IgG antibodies 5,920 R
- Examination during pregnancy. 1st trimester 16,690 RUR
- Entry into IVF RUB 23,020
Research result
The test is qualitative. The result is given in terms of “negative”, “doubtful”, “positive”.
Positive – IgG antibodies to Toxoplasma gondii were detected. This may indicate the initial period of a primary infection, or an infection suffered earlier. To differentiate these conditions, it is advisable to conduct a repeat study after 3-4 weeks. An increase in the resulting coefficient (CP) by 4 or more times indicates an active infection.
Doubtful - the result obtained is on the border of the threshold value, which does not allow reliably (with a probability of more than 95%) to classify the result as “Positive” or “Negative”. It should be borne in mind that such a result is possible with a very low level of antibodies, which can occur, in particular, in the initial period of the disease. Depending on the clinical situation, repeat testing of antibody levels after 3-4 weeks may be useful to assess dynamics. If a second questionable result is obtained, the sample is considered negative, that is, no antibodies are detected.
Negative - no IgG antibodies were detected in the test sample. This may indicate no contact of the animal with Toxoplasma gondii or early infection. Depending on the clinical situation, repeat testing of antibody levels after 3-4 weeks may be useful.
Detailed description of the study
Toxoplasma gondii is an intracellular parasite belonging to the kingdom of protozoa. There are three main infectious stages in the life cycle of this pathogen: sporozoites, tachyzoites and bradyzoites.
Cats and other members of the feline family serve as the final host of Toxoplasma. The parasite lives in the cells of their digestive system. Cats become infected by eating contaminated meat.
Living in the intestines of these animals, Toxoplasma produces eggs (oocysts) that are resistant to environmental influences. In this form, the parasite is excreted in the feces of cats. It enters the gastrointestinal tract of a person, who is the intermediate host of this parasite, when unwashed hands or surfaces come into contact with the oral cavity. This is possible in case of poor hygiene:
– while cleaning the cat litter; – after cutting infected meat; – contact with soil containing Toxoplasma eggs.
Entry into the human intestine promotes the transformation of the parasite into tachyzoites. Tachyzoites are a rapidly reproducing form of the parasite that characterizes the acute stage of infection. In this form, Toxoplasma spreads throughout the body through the bloodstream, including the muscles and brain. Subsequently, this parasite turns into a slowly reproducing form - bradyzoites. It is this that prevails in chronic infection.
Tissue cysts containing viable bradyzoites persist for years throughout a person's life. The brain and muscles are the most common sites of chronic latent infection, although they can be found in the lungs, liver, kidneys, and other internal organs.
Infection with toxoplasmosis has no characteristic symptoms and is usually similar to manifestations of an acute respiratory infection or mononucleosis. The deterioration in well-being is short-term, sometimes the disease occurs in an erased form or is asymptomatic. The infection is suppressed by the immune system, but the pathogen can remain in the body for life. Reactivation of toxoplasmosis occurs in case of immune suppression, for example, during infection with the human immunodeficiency virus.
Toxoplasma infection is dangerous for pregnant women. In some of them, this parasite is able to overcome the placental barrier and infect the fetus. In the first trimester of pregnancy, the risk of exposure to the fetus is lower (about 20%), while by the end of the third trimester it reaches 80%. However, infection in the early stages of pregnancy leads to more severe consequences: spontaneous abortion, hydrocephalus or mental retardation of the child. At the same time, in the last trimester, most cases are milder, but sometimes lead to eye damage and blindness.
Infection with Toxoplasma leads to the formation of class M antibodies by the immune system, then class G. The latter reflect contact with the pathogen over a period of more than a month. Analysis of the amount of IgG is necessary to assess immunity to Toxoplasma, which is especially important for people with compromised immunity and during pregnancy.
Ultrasound Gynecologist
Toxoplasmosis is a widespread parasitic infection, characterized by a variety of clinical manifestations and significant variability in the course of the process - from asymptomatic carriage to severe and fatal forms of the disease. A feature of toxoplasmosis is the possibility of infection of the fetus during intrauterine development with the occurrence of severe developmental anomalies. In different regions of the world, the infection rate of the population with toxoplasmosis ranges from 10 to 90%.
ETIOLOGY
The causative agent of toxoplasmosis, Toxoplasma gondii, is an intracellular parasite, 4–7 microns in size, capable of parasitizing in humans and animals in almost all organs and tissues.
The main source of infection is representatives of the cat family, in whose body the pathogen undergoes a full development cycle (tissue and intestinal) and is excreted in the form of oocysts with feces. Over the course of 10–15 days of illness, an infected cat releases about 2 billion oocysts into the external environment. In soil, oocysts can remain viable for up to 1.5–2.0 years.
Ways of infection with toxoplasmosis:
• Oral – the main and most common method of infection is the ingestion of cysts, oocysts when consuming raw or insufficiently heat-treated meat products with toxoplasma cysts in them, poorly washed greens, vegetables, fruits contaminated with oocysts of the pathogen.
• Percutaneous – for skin wounds, damage to mucous membranes in workers of slaughterhouses, meat processing plants, and veterinarians.
• Transplacental – when parasitemia occurs during pregnancy.
• Blood transfusion, transplantation - with transfusion of infected blood and organ transplantation.
PATHOGENESIS
During oral infection, pathogens penetrate the epithelial cells of the small intestine, where they multiply, then penetrate into the regional lymph nodes, and from them, through the lymph flow, into the blood. Dissemination of the pathogen leads to damage to a wide variety of organs and tissues. The formation of immunity contributes to the disappearance of the pathogen from the blood, and its reproduction in cells stops. True tissue cysts are formed, which can persist in the body for a long time, for decades.
CLINIC
The majority of those infected with Toxoplasma gondii (95–99%) have no clinical manifestations of the disease. Carriage of the parasite is observed, accompanied by a stable level of concentration of class G immunoglobulins (Toxo-IgG) in the blood. Carriage does not require any therapeutic measures, and the carrier should be regarded as a practically healthy person.
At the same time, clinically pronounced variants of the course of the primary infection (usually occurring in persons with impaired immunity) with the development of encephalitis, myocarditis, myositis, uveitis (chorioretinitis), and the formation of chronic toxoplasmosis are also possible.
Reactivation of a latent infection is possible (1–5% of the number of infected) under the influence of factors that can cause immunosuppression (influenza, acute respiratory infections, long-term treatment with cytostatics, glucocorticoids, radiation therapy, psycho-emotional stress).
The problem of generalization of latent toxoplasmosis in HIV-infected people with the development of severe necrotizing encephalitis with a high probability of death is very relevant. Toxoplasmosis is a characteristic and frequently observed AIDS-associated infection, leading to damage to various organs and systems in patients.
TOXOPLASMOSIS IN PREGNANT WOMEN
Toxoplasmosis is included in the group of TORCH infections, which are considered potentially dangerous for the intrauterine development of the fetus.
According to modern concepts, intrauterine damage to the fetus due to toxoplasmosis can occur only in the case of primary infection during pregnancy. During parasitemia, infection of the placenta and transmission of Toxoplasma gondii to the fetus is possible. Usually in such cases there is a threat of miscarriage; Modern highly effective methods of treating threatened miscarriages make it possible to save this pregnancy, but it can result in the birth of a child with a severe intrauterine infection.
In the case of primary infection of a pregnant woman, the risk of intrauterine infection of the fetus increases from approximately 17% in the first trimester (placental permeability is minimal) to 80% in the third trimester (placental permeability increases). The severity of fetal damage depends on the stage of intrauterine development. Infection in the 1st–2nd trimester of pregnancy leads to the most severe consequences (Table 1).
| Table 1 Risk of fetal infection with primary toxoplasmosis | ||
| Gestation period, weeks | Risk of infection, % | Exodus |
| 0–8 | 17 | Severe developmental anomalies |
| 8–18 | 25 | Damage to the central nervous system (hydro-, microcephaly, calcifications in brain tissue, episyndrome), chorioretinitis, microphthalmia |
| 18–24 | 65 | Impaired functions of various organs (hepatosplenomegaly, jaundice, anemia, thrombocytopenia) |
| 24–40 | 80 | Subclinical manifestations of the disease with manifestation after several years (deafness, chorioretinitis, impaired psychomotor development) |
Thus, it is important to determine the time of infection of a pregnant woman: long before, immediately before or during pregnancy.
We can confidently talk about infection during pregnancy if a set of the following indicators is available: a) determination of seroconversion; b) a twofold increase in the concentration of specific IgG when studying paired sera taken at an interval of 2–3 weeks and the simultaneous presence of specific IgM, IgA; c) the presence of low-avidity specific IgG.
The later a pregnant woman goes to the antenatal clinic and the later the initial serological examination is carried out, the less definite an answer can be given regarding the timing of infection.
Only with confirmed infection in the first trimester of pregnancy, when the risk of giving birth to a child with gross organic lesions of the central nervous system and eyes is greatest, can the question of terminating the pregnancy for medical reasons be raised! Women infected in the second and third trimester of pregnancy are subject to treatment.
When giving recommendations for subsequent pregnancies, it is necessary to take into account that a child with congenital toxoplasmosis can only be born once in a lifetime. During subsequent pregnancies, a woman may not have to worry about giving birth to a child with congenital toxoplasmosis.
A woman should consult a doctor and undergo a laboratory examination for all TORCH infections 2–3 months before a planned pregnancy, since only in this case is it possible to identify a risk group (seronegative) and take appropriate therapeutic or preventive measures.
LABORATORY DIAGNOSTICS
Due to the absence of pathognomonic symptoms, making a diagnosis of primary toxoplasmosis based on the clinical picture is practically excluded, so laboratory diagnostic methods play a decisive role: determination of specific antibodies and identification of the pathogen itself.
Currently, serological methods, mainly ELISA, are most widely used to diagnose toxoplasmosis, since methods for detecting Toxoplasma gondii have limitations - the pathogen is present in the blood for a short time.
When Toxoplasma enters the human body, after 7–14 days the primary humoral immune response begins: specific IgM (Toxo-IgM) is produced. The maximum level of Toxo-IgM concentration is reached by the 20–30th day from the onset of infection. Their complete disappearance in most cases (about 70%) occurs within 3–4 months, however, Toxo-IgM may be present for a longer period - up to 1 year or more (about 10% of cases).
Reinfection with Toxoplasma against the background of a previously acquired healthy carriage can also lead to the appearance of Tocho-IgM. Currently, there is no confirmed data that Tocho-IgM can be detected during reactivation of a latent infection, chronic toxoplasmosis.
Toxo-IgA begins to be detected in the blood 14 days after infection, reaching its maximum concentration after a month. Toxo-IgA usually disappears after 6 months (about 90% of cases), but can persist in some cases for more than 1 year. Therefore, the detection of Toxo-IgM and Toxo-IgA is not a strict indicator of a “fresh” infection, but only indicates a primary infection within the previous 12 months.
The level of concentration of specific immunoglobulins of class G (Toxo-IgG) increases in the first 2–3 months of the disease, during the year their concentration remains stable, and then decreases slightly.
To obtain more accurate data on the timing of infection and the duration of the infectious process, the determination of the Toxo-IgG avidity index is used. Avidity is a characteristic of the strength of binding of specific antibodies to the corresponding antigens. When an immune response is formed, Toxo-IgGs are first formed, which have low avidity, i.e. quite weakly binding to the antigen.
The avidity index (AI) of Toxo-IgG increases in the first 2–6 months of the disease. If low avidity IgG is detected in the blood, along with specific Toxo-IgM and Toxo-IgA, this indicates an acute stage of the primary infection. The presence of Toxo-IgM, Toxo-IgA and high-avidity IgG suggests long-term persistence of these immunoglobulins after the completion of the acute stage of the primary infection or a secondary immune response in the case of reinfection with T. gondii. Determination of high-avidity IgG in the absence of IgM indicates pastinfection.
Detection of low-avidity IgG with a negative result for Toxo-IgM can occur after infection for more than 3 months. In such cases, it is necessary to determine the dynamics of the increase in the avidity index.
In immunodeficient patients, an increase in the concentration of Toxo-IgG and the detection of Toxo-IgM and Toxo-IgA are rarely observed. Proof of the presence of infection can be the detection of T. gondii, in particular, the DNA of the pathogen using PCR.
The probable duration of T. gondii infection can be determined by analyzing the full range of serological markers of toxoplasmosis:
results of detection of specific IgM, IgA,
determination of the IgG avidity index and IgG concentration over time (Table 2).
DIAGNOSTICS OF CONGENITAL TOXOPLASMOSIS
If congenital toxoplasmosis is suspected, serological examination of the mother and child should be carried out at least 4–6 times during the first year of the child’s life. As is known, class G immunoglobulins (as opposed to IgM) can be transferred to the fetus transplacentally. In the absence of infection of the fetus by 4–6 months. After birth, the concentration of Tocho-IgG decreases sharply due to the breakdown of maternal antibodies. In case of infection, the concentration of Tocho-IgG increases, but in the first half of life it is “masked” by the presence of maternal antibodies.
The most accurate criterion for the presence of congenital toxoplasmosis is the detection of Tocho-IgM in the child’s blood serum. However, due to the immaturity of the newborn’s immune system and low production of IgM, as well as the possibility of the acute stage of infection occurring in utero (if infected in early pregnancy), the fact of infection is not always confirmed by the presence of Tocho-IgM in the umbilical cord blood. Thus, the absence of Tocho-IgM in newborns and young children does not provide grounds to exclude intrauterine infection with toxoplasmosis.
Since IgA does not cross the placenta, determination of Tocho-IgA can help in the diagnosis of fetal infection, neonatal and postnatal monitoring of congenital toxoplasmosis. The analysis of the child’s clinical condition, the mother’s obstetric history, and confirmation of the diagnosis by detecting T. gondii DNA in the child’s blood and urine using PCR is of decisive importance.
Means of immunoprophylaxis for toxoplasmosis have not been developed to date, therefore, only determining the immune status of pregnant women in relation to toxoplasma infection makes it possible to control the development of primary infection with T. gondii, which is very dangerous for the fetus.
Avidity (Latin - avidity) is a characteristic of the strength of the connection of specific antibodies with the corresponding antigens. During the body's immune response to the penetration of an infectious agent, a stimulated clone of lymphocytes begins to produce first specific IgM antibodies, and somewhat later specific IgG antibodies. IgG antibodies initially have low avidity, that is, they bind the antigen quite weakly. Then the development of the immune process gradually (this can be weeks or months) moves towards the synthesis by lymphocytes of high-avidity IgG antibodies, which bind more firmly to the corresponding antigens. High avidity of specific IgG antibodies allows one to exclude recent primary infection.
Confirming or excluding the fact of a recent primary infection with Toxoplasma gondii is especially important when examining pregnant women, since the risk of fetal pathology is significantly increased with acute primary infection during pregnancy, compared with chronic infection and reactivation of latent infection. Therefore, there is a constant search for new diagnostic approaches that allow the most reliable assessment of the stage and form of the infectious process. The use of IgG antibody avidity as an indicator of the duration of primary infection, first proposed by Finnish researchers (Hedman KM et al., 1989), has now been introduced into the practice of serological studies for TORCH infections in a number of countries. Thus, in France, where, as in Russia, the problem of toxoplasmosis is still relevant, this test is included in the mandatory examination algorithm for suspected toxoplasmosis in pregnant women.
The detection in serum of the presence of both IgG and IgM antibodies to an infectious agent can be interpreted as evidence of a recent primary infection, since, as is known, the disappearance of IgM antibodies is usually about 3 months from the onset of the infectious process. But the period of circulation of IgM antibodies can vary significantly depending on the infectious pathogen and the individual characteristics of the body’s immune response. When infected with Toxoplasma gondii, trace amounts of IgM antibodies to these infectious agents are in some cases detected for 1-2 years or more.
Thus, their presence in the blood of a pregnant woman does not always confirm primary infection during pregnancy. In addition, the specificity of even the best commercial test systems for detecting IgM antibodies is not absolute. In some situations, as a consequence of the very high sensitivity of the tests, nonspecific false-positive results are possible. Detection of high-avidity IgG antibodies in the blood in this situation allows one to exclude recent primary infection. Low-avidity IgG antibodies, on average, are detected within 3-5 months from the onset of infection (this may depend to some extent on the method of determination), but are sometimes produced over a longer period. In itself, the detection of low-avidity IgG antibodies is not an unconditional confirmation of the fact of fresh infection, but serves as additional confirmatory evidence among other serological tests. When the infection is reactivated, specific high-avidity IgG is detected.
Treatment
Most cases of acquired infection in immunocompetent individuals resolve without specific therapy. For choreoretinitis or damage to vital organs, a combination of pyrimethamine and sulfadiazine is prescribed. An alternative may be a combination of pyrimethamine with clindamycin if sulfadiazine is poorly tolerated. In the treatment of choreoretinitis and central nervous system lesions, glucocorticoids are used. HIV-infected patients with encephalitis should receive lifelong suppressive therapy to prevent recurrence of infection.
For severe and asymptomatic congenital infection, a combination of pyrimethamine with sulfadiazine and folic acid is recommended as initial therapy. Therapy is usually long-term, sometimes up to 1 year. Treatment of toxoplasmosis that occurs during pregnancy, including in HIV-infected women, should be carried out with spiramycin. When a woman is infected in the third trimester or a fetus is infected after 17 weeks of gestation, a combination of pyrimethamine and sulfadiazine is used.
Telithromycin has been shown to have high in vitro activity against T. gondii (M. Kilinc, M. Hokelek, M. Erturk).
Animal studies have shown that atovaquone in combination with pyrrolidin dithiocarbamate causes the conversion of tachyzoites to the tissue cyst stage (O. Djurkovic-Djakovic, A. Nikolic, B. Bobic, I. Klun).
Prevention
Pregnant women whose serostatus is unknown or who are seronegative should avoid contact with soil and other objects that may be contaminated with cat feces, or wear gloves and wash their hands after handling. To avoid infection, domestic cats should not eat raw meat or caught rodents. It is necessary to carry out sufficient heat treatment of meat, wash vegetables and fruits, wash hands and kitchen surfaces after contact with raw meat, vegetables and fruits.
Indications for use
1. Screening examination before and during pregnancy.2. Lymphadenopathy during pregnancy, if immunity to toxoplasma was previously absent or not studied.3. Signs of intrauterine infection, feto-placental insufficiency.4. Encephalitis/meningoencephalitis due to HIV infection, neoplastic diseases, taking cytostatic drugs, and other immunodeficiency states.5. Lymphadenopathy of unknown origin6. Hepatosplenomegaly of unknown origin.7. Fever of unknown origin.8. Suspicion of congenital toxoplasmosis.
Causes of toxoplasmosis
The single-celled parasite, which is the causative agent of toxoplasmosis, penetrates the body not only of humans, but also of various animals and birds, which are considered intermediate hosts. To go through the full development cycle, and most importantly to reproduce, the parasite needs the intestines of a cat, so any infected member of the cat family is its main host. The infection in the oocyst stage enters the environment along with feces and remains active for a long time (1-2 months). It must be said that cats excrete oocysts once in their lives and usually during the age of kittens. Therefore, the following possible routes of infection for humans are:
- from the environment - directly from a sick cat (by contact with its saliva and blood, if there are scratches or abrasions on the skin, when cleaning the tray), as well as when working with soil that may be contaminated with oocysts;
- eating contaminated foods (animal meat that has been improperly or poorly cooked, raw eggs and milk, dirty vegetables, fruits);
- during pregnancy, the mother can infect the fetus;
- in isolated cases, infection is possible during transplant operations.
Toxoplasma, penetrating various human tissues, where it can remain in a dormant form in the form of cysts (latent infection) throughout life. A healthy immune system responds to this with little to no symptoms or signs of a mild cold. But with a decrease in immunity, an exacerbation of infection occurs. Microorganisms are carried through the bloodstream to internal organs, affecting nerves, muscles, and the heart. This disease can have acute, chronic and asymptomatic forms, acquired or congenital. Risk groups include people with weak immune systems and pregnant women.
Toxoplasmosis (quantitative method)
Toxoplasmosis (quantitative method)
A chronic parasitic disease that causes, in people with reduced immunity, damage to the nervous system, heart and other organs. The causative agent is Toxoplasma. The source of infection is cats and some wild felines. The route of transmission is nutritional. Serological tests for anti-Toxoplasma IgG may indicate Toxoplasma carriage (at low titers) or acute infection (at high titers). Determination of Toxoplasma IgM is used to identify congenital toxoplasmosis or fresh infection (in children and adults). An increase in the titer of specific antibodies by 4 or more times has diagnostic significance. If the concentration of Toxo IgG in the analyzed sample is 10 IU/ml or lower, the result is assessed as negative. If the Toxo IgG concentration is 40 IU/ml or higher, the result is assessed as positive. In case of a positive ELISA result for Toxo IgG in pregnant women, it is important to determine the duration of infection (exclude acute toxoplasmosis).
Microplate washer . Stat Fax 2600 Washing machine for ELISA. Designed for washing 96-well plates used in ELISA or EIA applications, including clinical diagnostic assays that require plate washing. Manufactured by AWARENESS TECHNOLOGY INC, USA
To do this, the serum is analyzed for Toxo IgM content, the Toxo IgG avidity index is determined, and the dynamics of the development of the immune response are studied.
To clarify the diagnosis, you MUST pass:
- general blood analysis; — to detect antibodies (IgG, IgM) to Toxoplasma gondii in plasma or serum using ELISA; If the result for Toxo IgM is negative and the Toxo IgG avidity index is more than 50%, pregnant women without clinical indications do not need to be examined for toxoplasmosis in the future. If the result is positive for Toxo IgM, the patient’s blood serum is left frozen. After 10-15 days, blood is taken again and a Toxo IgG test is performed on both blood samples in comparison . An increase in Toxo IgG concentration over time by 1.5 times or more indicates infection with Toxoplasma gondii within the previous 2 to 3 months. The presence of low-avidity Toxo IgG confirms recent infection. When the Toxo IgG concentration is from 10 to 40 IU/ml, the result is considered intermediate. An intermediate result may also indicate the beginning of the development of an immune response during the acute stage of the disease. Therefore, sera with an intermediate result for Toxo IgG must be examined as potentially positive - in dynamics for Toxo IgG and IgM. — CONTACT YOUR DOCTOR IMMEDIATELY. - after treatment, it is imperative to undergo control tests to the extent that the disease was diagnosed.
Preparing for the study
Requirements for samples: For cats: - a smear from the rectum (in an Eppendorf tube with 0.5 ml of saline solution) + venous blood in a tube with EDTA (at least 1 ml) or plasma (at least 300 μl) - for cases when the cat itself becomes an intermediate host and exhibits appropriate symptoms - blood with EDTA, minimum volume - at least 2 ml - when neurological symptoms appear, a smear from the mucous membranes of the eyes + blood with EDTA For dogs: - a smear from the mucous membranes of the eyes + blood with EDTA - blood with heparin is not suitable for PCR studies! It is STRICTLY prohibited: leaving cotton probes in the swab tube when submitting samples to the laboratory. Storage of samples before delivery to the laboratory: - up to 48 hours at +2-+8⁰С
Take a blood test for toxoplasmosis
Toxoplasmosis is a parasitic infectious anthropozoonotic disease caused by Toxoplasma gondii. Toxoplasma has a long development cycle, the main stages of which occur in the body of animals, birds or humans. Also, Toxoplasma particles are resistant to environmental factors, so they can persist for a long time in feces, soil, and sand. The main route of infection is fecal-oral and transplacental. Since the final host of parasites is animals, often cats, special attention is paid to contact with them. In turn, the risk of infection for cats increases when they come into contact with rodents. Toxoplasma is released into the environment through cat feces, which contributes to human infection through contact with an animal or a place contaminated with feline fecal particles.
Entry into the human body usually remains unnoticeable. Most often, the disease occurs without a pronounced clinical picture, or catarrhal phenomena and symptoms of intoxication are possible, which is more often observed in people with reduced immunity.
A person can be a carrier of Toxoplasma for a long time and not realize it. Usually the fact is confirmed by laboratory diagnostics. Since carriage of Toxoplasma is of particular importance during pregnancy, it is extremely important to undergo a screening examination already during the planning period, which reflects the presence of antibodies of various classes to Toxoplasma. This is necessary to exclude the possibility of infection of the fetus, because Toxoplasma poses the greatest danger to the intrauterine development of the baby. It is during pregnancy that it can cause irreversible changes in the structure of the fetal organs, leading to defects and pregnancy loss. This danger appears only during primary infection. Therefore, diagnosis of toxoplasmosis should be carried out both before pregnancy and during it, for up to 12 weeks.
The examination includes the determination of class M and G antibodies to Toxoplasma gondii. In response to the introduction of the parasite, the body begins
produce class M antibodies, which appear in the blood after about 2 weeks and persist for up to 8-10 weeks. After 8 weeks from the onset of infection, class G antibodies begin to be released into the blood, which are considered to be antibodies of the disease. The presence of G antibodies without M antibodies only indicates that the body has previously encountered toxoplasma, but at the moment the person is not sick and treatment is not required. This information is extremely important at the pregnancy planning stage, during pregnancy. After all, the presence of Ig G provides immunity to toxoplasma and eliminates re-infection, which is extremely dangerous during pregnancy. Antibodies are determined using the ICL method, which relates to serological diagnostics and is an objective study in the complex diagnosis of toxoplasmosis.
Typically, the determination of class G antibodies is carried out in combination with the determination of class M antibodies, as well as the avidity index, which makes it possible to estimate the duration of infection and the stage of the disease.
In newborns, the presence of class G antibodies may indicate their transmission from the mother and do not indicate intrauterine infection.
Requirements for biomaterial
Intravital diagnosis of clinical toxoplasmosis is based on the following combination: 1. Fourfold or more increase, decrease in Ig G titer (in paired sera, for 2 weeks) for the acute stage 2. Exclusion of other causes of the clinical syndrome 3. Response to chemotherapy Early: duration – up to 1 year. Positive Ig M, and by the 3rd week positive Ig G specific antibodies appear (with a peak at 2-4 months). Peak concentration of Ig M: end of the first, second month, blood PCR is positive. A negative result of only a PCR test of blood or cerebrospinal fluid does not exclude toxoplasmosis either in a clinically ill or in an asymptomatic animal. A negative result for Ig G and PCR in a clinically sick animal proves the absence of toxoplasmosis. Changes in routine laboratory tests are more typical for acute systemic toxoplasmosis. Additional studies: CBC: non-regenerative anemia, eosinophilia, neutrophilia, less often lymphomonocytosis. In severe cases in cats there is leukopenia with a shift to the left. Blood biochemistry: changes depend on damaged systems. CSF: increased protein levels, pleocytosis (mononuclear). Hypoproteinemia, hypoalbuminemia. Late: 1 year after the onset of the disease. Positive Ig G specific antibodies. Blood PCR is negative. Kittens: born from a cat with chronic toxoplasmosis have maternal Ig G within 8-12 weeks after birth