Infiltrative pulmonary tuberculosis
Primary infection with Mycobacterium tuberculosis and latent course of tuberculosis infection. Primary infection of humans with MBT usually occurs through the aerogenous route. Other routes of penetration - nutritional, contact and transplacental - are much less common.
The respiratory system is protected from the penetration of mycobacteria by mucociliary clearance (secretion of mucus by goblet cells of the respiratory tract, which glues incoming mycobacteria, and further elimination of mycobacteria using wave-like vibrations of the ciliated epithelium). Violation of mucociliary clearance during acute and chronic inflammation of the upper respiratory tract, trachea and large bronchi, as well as under the influence of toxic substances, makes it possible for mycobacteria to penetrate the bronchioles and alveoli, after which the likelihood of infection and tuberculosis increases significantly. The possibility of infection through the nutritional route is determined by the condition of the intestinal wall and its absorption function.
The causative agents of tuberculosis do not secrete any exotoxin that could stimulate phagocytosis. The possibilities for phagocytosis of mycobacteria at this stage are limited, so the presence of a small amount of the pathogen in the tissues does not appear immediately. Mycobacteria are outside the cells and multiply slowly, and the tissues retain their normal structure for some time. This condition is called "latent microbiism." Regardless of the initial localization, they enter the regional lymph nodes with the lymph flow, after which they spread lymphogenously throughout the body - primary (obligate) mycobacteremia occurs. Mycobacteria are retained in organs with the most developed microvasculature (lungs, lymph nodes, renal cortex, epiphyses and metaphyses of tubular bones, ampullar-fimbryonic sections of the fallopian tubes, uveal tract of the eye). Since the pathogen continues to multiply, and immunity has not yet formed, the population of the pathogen increases significantly. However, at the site of accumulation of a large number of mycobacteria, phagocytosis begins. First, the pathogens begin to phagocytose and destroy polynuclear leukocytes, but to no avail - they all die when they come into contact with the office due to their weak bactericidal potential.
Then macrophages are involved in the phagocytosis of MBT. However, MBT synthesize ATP-positive protons, sulfates and virulence factors (cord factors), as a result of which the function of macrophage lysosomes is disrupted. The formation of a phagolysosome becomes impossible, so the lysosomal enzymes of macrophages cannot act on the engulfed mycobacteria. MBTs are located intracellularly, continue to grow, multiply and increasingly damage the host cell. The macrophage gradually dies, and mycobacteria again enter the intercellular space. This process is called "incomplete phagocytosis".
Acquired cellular immunity Acquired cellular immunity is based on the effective interaction of macrophages and lymphocytes. Of particular importance is the contact of macrophages with T helper cells (CD4+) and T suppressor cells (CD8+). Macrophages that have absorbed MBT express mycobacterial antigens on their surface (in the form of peptides) and release interleukin-1 (IL-1) into the intercellular space, which activates T-lymphocytes (CD4+). In turn, T helper cells (CD4+) interact with macrophages and perceive information about the genetic structure of the pathogen. Sensitized T-lymphocytes (CD4+ and CD8+) secrete chemotaxins, gamma-interferon and interleukin-2 (IL-2), which activate the migration of macrophages towards the location of the office, increase the enzymatic and general bactericidal activity of macrophages. Activated macrophages intensively produce reactive oxygen species and hydrogen peroxide. This is the so-called oxygen explosion; it acts on the phagocytosed tuberculosis pathogen. With simultaneous exposure to L-arginine and tumor necrosis factor-alpha, nitric oxide NO is formed, which also has an antimicrobial effect. As a result of all these processes, the destructive effect of MBT on phagolysosomes is weakened, and the bacteria are destroyed by lysosomal enzymes. With an adequate immune response, each subsequent generation of macrophages becomes more and more immunocompetent. Mediators released by macrophages also activate B-lymphocytes, which are responsible for the synthesis of immunoglobulins, but their accumulation in the blood does not affect the body’s resistance to MBT. But the production of opsonizing antibodies by B lymphocytes, which envelop the mycobacteria and promote their adhesion, is useful for further phagocytosis.
An increase in the enzymatic activity of macrophages and their release of various mediators can lead to the appearance of delayed-type hypersensitivity cells (DSHT) to MBT antigens. Macrophages transform into epithelioid Langhans giant cells, which are involved in limiting the area of inflammation. An exudative-productive and productive tuberculous granuloma is formed, the formation of which indicates a good immune response to the infection and the body’s ability to localize mycobacterial aggression. At the height of the granulomatous reaction in the granuloma there are T-lymphocytes (prevail), B-lymphocytes, macrophages (carry out phagocytosis, perform affector and effector functions); macrophages gradually transform into epithelioid cells (carry out pinocytosis, synthesize hydrolytic enzymes). In the center of the granuloma, a small area of caseous necrosis may appear, which is formed from the bodies of macrophages that died upon contact with the office.
The PCI reaction appears 2-3 weeks after infection, and fairly pronounced cellular immunity is formed after 8 weeks. After this, the proliferation of mycobacteria slows down, their total number decreases, and the specific inflammatory reaction subsides. But complete elimination of the pathogen from the source of inflammation does not occur. Preserved MBTs are localized intracellularly (L-forms) and prevent the formation of phagolysosomes, therefore they are inaccessible to lysosomal enzymes. Such anti-tuberculosis immunity is called non-sterile. The MBT remaining in the body maintains the population of sensitized T-lymphocytes and provides a sufficient level of immunological activity. Thus, a person can retain MBT in his body for a long time and even throughout his life. When immunity is weakened, there is a threat of activation of the remaining MBT population and tuberculosis disease.
Acquired immunity to MBT decreases with AIDS, diabetes, peptic ulcers, alcohol abuse and long-term drug use, as well as with fasting, stressful situations, pregnancy, treatment with hormones or immunosuppressants.
In general, the risk of developing tuberculosis in a newly infected person is about 8% in the first 2 years after infection, gradually decreasing in subsequent years.
The emergence of clinically manifest tuberculosis In the case of insufficient activation of macrophages, phagocytosis is ineffective, the proliferation of MBT by macrophages is not controlled and therefore occurs in geometric progression. Phagocytic cells cannot cope with the amount of work and die en masse. At the same time, a large number of mediators and proteolytic enzymes enter the intercellular space, which damage adjacent tissues. A kind of “liquefaction” of tissues occurs, a special nutrient medium is formed that promotes the growth and reproduction of extracellularly located MBT.
A large MBT population upsets the balance in immune defense: the number of T-suppressor cells (CD8+) increases, the immunological activity of T-helper cells (CD4+) decreases. First, PCT to MBT antigens sharply increases and then weakens. The inflammatory reaction becomes widespread. The permeability of the vascular wall increases, plasma proteins, leukocytes and monocytes enter the tissue. Tuberculous granulomas are formed, in which caseous necrosis predominates. Infiltration of the outer layer with polynuclear leukocytes, macrophages and lymphoid cells increases. Individual granulomas merge, and the total volume of tuberculosis lesions increases. Primary infection transforms into clinically manifest tuberculosis.
In what diseases does ground glass appear on tomograms?
According to the Department of Radiation Diagnostics of the MMA named after. Sechenov, the picture of “dullness” is most often given by pneumonia (viral, bacterial, fungal), however, lung infiltration is also characteristic of other pathological conditions. Here are just a few of them:
- Alveolar proteinosis;
- Pneumonitis;
- Hemorrhage;
- Granulomatosis;
- Alveolar edema;
- Allergic alveolitis;
- Tuberculosis;
- Drug-induced lung damage;
- Pulmonary infarction;
- Viral infection;
- Dermatomyositis;
- Tumors;
- Rheumatoid arteritis;
- Respiratory bronchiolitis with ILD;
- Sjogren's syndrome;
- Goodpasture's syndrome;
- Scleroderma.
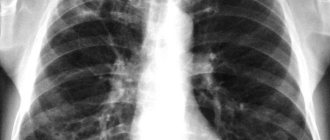
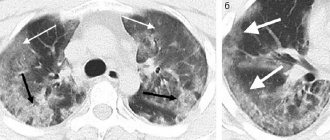
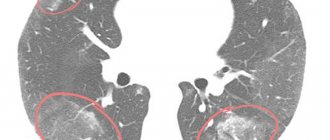
Assessing CT data of the lungs, a radiologist differentiates diseases according to the specific pattern of “ground glass”: their number, location, and the presence of other signs by which it is possible to determine the cause of pathological changes in lung tissue. For example, bilateral viral pneumonia is characterized by the presence of “ground glass” lesions located peripherally in the lower and posterior parts of the lungs. At a later stage, consolidation of foci of infiltration with thickening of the alveolar septa is observed.
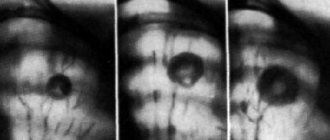
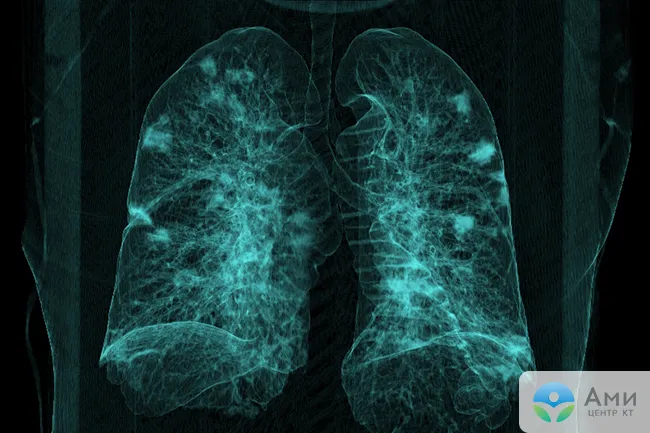
“Frost glass” in tuberculosis can be located next to the bronchi or disseminated - in this case, there are many small light areas (infiltrates) randomly located throughout the lung cavity. Also, with tuberculosis, a CT scan of the lungs can detect characteristic fibrous strands leading to the root of the lung - this is peribronchial inflammation, signs of lymphostasis with enlarged lymph nodes. Single small ground-glass areas without a clear pattern of any disease may indicate a neoplasm, developing fibrosis, or adenocarcinoma (cancer) of the lungs.
Sometimes “frosted glass” indicates vascular pathologies that lead to compression of the alveolar space. In obese patients, it may appear in the gravity-dependent (lower) areas of the lungs under the influence of excess weight. In healthy patients, the “ground glass” effect may appear on tomograms if the chest scan is performed while exhaling (correctly, during a deep inhalation).
Without taking these errors into account and speaking about the true “ground glass” syndrome on a CT scan of the lungs, we note that this is a sign of non-functional areas of the lungs. Normally, there should be no compactions or obstructions to breathing, and the lung tissue is presented on tomograms as a uniform dark color. Very rarely, “frosted glass” indicates the individual characteristics of the patient’s body, for example, the atypical location of the diaphragm in newborns. But these are isolated cases, so let's look at the most common patterns of diseases in which “ground glass” is found on tomography.
“Frost glass” on CT scan for pneumonia
Most often, “ground glass” on a CT scan appears with pneumonia, and it can be absolutely any type of pneumonia: viral, bacterial, with an atypical development of the symptom complex, very rare interstitial, and so on. Since the spectrum of pneumonia pathogens is extremely wide, and it is not always possible to differentiate lung diseases based on the “ground glass” effect alone, the patient is recommended to undergo laboratory diagnostics - a blood test or respiratory discharge for mycoplasmas, pneumococci, coronavirus and other pathogens.
A common sign of acute pneumonia on CT is the presence of infiltrates (“ground glass”) of various shapes and lengths. “Frosted glasses” are located around lesions or diffusely, as in tuberculosis. However, unlike tuberculosis, their size is usually larger, there is a tendency to consolidate infiltrates and form a “cobblestone” pattern. In some cases, the lumens of the bronchi containing gas are visualized. This is called "air bronchography" syndrome. Combined with the frosted glass effect, it is also a clear sign of pneumonia.
Pneumonia caused by the coronavirus COVID-19 is characterized by a peripheral location of lesions under the pleura. The bilateral lower lobes and posterior sections of the lungs are most vulnerable. There is a tendency towards consolidation of “ground glass” and thickening of the alveolar septa, and sometimes there are signs of pulmonary fibrosis.
Severe forms of coronavirus pneumonia are accompanied by acute respiratory distress syndrome. ARDS is an extensive bilateral inflammation in which multiple infiltrates and pulmonary edema are observed. On tomograms, fragmentary areas of “ground glass” cortical shape are present on both sides and have the appearance of a “patchwork quilt”.
With Pneumocystis pneumonia, caused by the yeast-like fungus Pneumocystis Jirovecii, a slightly different picture is observed. Areas of ground-glass compaction of the lungs are usually located on both sides symmetrically (but sometimes diffusely and unevenly). Compactions predominate in the hilar areas of the lungs, and diffuse changes - in the upper and lower sections. Pneumocystis pneumonia, as well as viral pneumonia associated with COVID-19, is characterized by a consolidation effect and a “patchwork quilt” symptom, but other signs are also visible on tomograms of the lungs: air cysts, pneumothorax.
A separate group of diseases is represented by idiopathic interstitial pneumonia, the cause of which cannot be determined. In addition to “ground glass”, CT scans of the lungs can detect the “honeycomb lung” symptom, bronchiectasis, and reticular changes. Idiopathic pneumonia requires histological examination.
Based on computed tomography data of the lungs and the patient’s medical history, the radiologist will be able to determine lung damage characteristic of pneumonia. As part of the differential diagnosis, the density and shape of the “frosted glasses”, their number are taken into account, and the pattern is assessed as a whole. However, the causative agent of pneumonia and treatment tactics can be determined after additional laboratory diagnostics.