Why is hemoglobin needed?
Almost all types of vertebrate animals require a special delivery system to transport oxygen to tissues, since molecular oxygen is poorly soluble in water: only 3.2 ml of O2 dissolves in 1 liter of blood plasma. The hemoglobin protein (Hb, Fig. 1) contained in vertebrate erythrocytes is capable of binding 70 times more - 220 ml O2/l. The Hb content in human blood varies between 120–180 g/l, which is twice as high as the concentration of plasma proteins (50–80 g/l). Therefore, hemoglobin makes the greatest contribution to maintaining the pH-buffering capacity of the blood. In its structure, adult human hemoglobin (HbA) is a tetramer consisting of two α- and two β-subunits with molecular masses of about 16 kDa. The α and β chains differ in amino acid sequence but have similar conformations.
Figure 1. Hemoglobin molecule. Hemoglobin is one of the most well studied proteins. It was discovered by the German physiologist Otto Funke in 1851, and the structure of this protein was described by the Austrian molecular biologist Max Perutz in 1959, for which he received the Nobel Prize in Chemistry three years later [1].
Visual Science
Figure 2. Oxygen saturation of hemoglobin and myoglobin
[2]
Each hemoglobin subunit carries a heme group with a ferrous iron ion at the center. When O2 binds to the iron atom in heme (Hb oxygenation) and O2 is removed (deoxygenation), the oxidation state of the iron atom does not change. The oxidation of Fe2+ to Fe3+ in heme is random. The oxidized form of hemoglobin, methemoglobin, is unable to transport O2. The proportion of methemoglobin is maintained by enzymes at a low level and amounts to 1–2% [2]. O2 binding centers on each of the four subunits act cooperatively: when an O2 molecule binds to one of them, the others increase their affinity for oxygen (this phenomenon is called positive cooperativity) [3]. As a result, the hemoglobin oxygen saturation curve has a pronounced sigmoidal character (Fig. 2, curve 2).
Another muscle protein, myoglobin, which is the evolutionary predecessor of hemoglobin, is a monomer and contains a single O2 binding site, which is why its oxygen saturation curve is non-sigmoidal (Fig. 2, curve 1). The affinity for oxygen of myoglobin is approximately 13 times higher than that of hemoglobin (50% saturation of myoglobin with O2 is achieved already at a partial pressure of oxygen of 1–2 mm Hg, while for hemoglobin this figure is 26 mm Hg. Art.) [4]. Because of this, hemoglobin is able to efficiently transfer oxygen to tissues and is a more efficient carrier than myoglobin. But it does not follow from this that myoglobin is an ineffective and poorly structured protein, since it performs a fundamentally different biological function - storing oxygen and providing it to mitochondria. These adaptive differences between myoglobin and hemoglobin arose as a result of millions of years of evolution...
What level of hemoglobin is considered normal?
For an adult man, the hemoglobin norm is 130-160 g/l of blood, for an adult woman - 120-140 g/l.
Photo: istockphoto.com
For children, the following values are considered normal:
- newborns up to two weeks of life - not lower than 150 g/l;
- children from two to four weeks of life - 120 g/l and above;
- children aged six to 59 months - 110-140 g/l;
- children aged from five to 11 years - 115-140 g/l;
- children aged 12 to 14 years - 120-150 g/l;
- children over 15 years old - 130-160 g/l.
Transparent fish
In 1927, an expedition of Norwegian whalers near Bouvet Island, during another commercial hunt, brought to land an unprecedented fish, almost colorless and, most interestingly, with transparent (“glass”) blood. It was the first vertebrate species discovered that did not contain the protein hemoglobin. Due to the striking resemblance of the fish's head to the head of a crocodile, the fish was named the crocodile whiteblood (Chaenocephalus aceratus). Whitefish (Channichthyidae; Fig. 3) or icefish live in cold waters near Antarctica and the southern coast of South America. The water temperature in these parts drops as low as −1.9 °C (the freezing point of sea water is lower than fresh water), and is quite constant.

[5–8]
Very few fish can survive the harsh conditions of Antarctica. Icefish survive due to a special antifreeze present in the blood that prevents the formation of ice crystals in the body. This antifreeze (AFGP, antifreeze glycoprotein) is a glycoprotein thought to be derived from pancreatic trypsinogen-like protease [9]. AFGP is able to bind to microscopic ice crystals and prevent their growth [10].
Icefish have a very low metabolic rate and spend most of their time practically motionless. Whitebloods live in oxygen-rich water and absorb it directly through the skin [11], because at low temperatures, blood containing hemoglobin becomes very viscous, and survival with such blood would be very problematic.
The lack of hemoglobin is compensated by modification of the cardiovascular system. All representatives of ice fish have a larger heart than other fish of the same size, and this increases stroke volume, increases the total amount of circulating blood several times and increases the speed of blood flow. At low blood pressure, this is achieved by reducing systemic resistance to flow. The combination of the high cardiovascular capacity, high oxygen content and relatively low metabolic rates of icefish allows sufficient oxygen to be supplied to the tissues [12].
Hemoglobin loss
White bloods survived the loss of hemoglobin genes quite a long time ago. Molecular analysis shows that in almost all icefish, one mutation led to the loss of the gene encoding the β chain and part of the α chain of hemoglobin. The loss of the ability to synthesize hemoglobin caused the development of compensatory changes: the volume of the heart and total blood volume increased (approximately 3.5 times compared to teleost fish of a similar size) [13–15]. Scientists, having analyzed the DNA of representatives of nototheniid fish, came to the conclusion that only one species of whitefish (Neopagetopsis iona) has hemoglobin genes, but they are not functional [16].
Along with hemoglobin, white bloods also lack myoglobin, which carries oxygen in skeletal muscles. Moreover, in ten species, myoglobin was preserved only in the cardiac muscle (in particular, in the ventricle) [17], and in six species, myoglobin was lost there as well, and the mechanism of gene loss is individual in each species [18]. A common mechanism for such loss is duplication of short (5–25 nucleotide) fragments, leading to a reading frame shift, premature termination of transcription, the appearance of a false polyadenylation signal, or impaired binding of RNA polymerase to the promoter region of DNA [19], [20].
The loss of hemoglobin was initially supposed to be an adaptation to cold: it is known that the solubility of oxygen in cold water is higher [21], which means that the need for hemoglobin, on the contrary, is less. The absence of red blood cells also reduces blood viscosity, which is especially critical in extremely low temperature conditions. During the process of evolution, whitebloods have undergone quite radical changes to compensate for the loss of hemoglobin, including twice the energy expenditure for pumping blood compared to other fish [22].
Ice fish evolved from a sedentary, bottom-dwelling ancestor. In the cold, well-mixed, oxygen-rich Antarctic waters, fish with low metabolic rates can survive even without hemoglobin. In the middle of the Tertiary period, the ecological crisis in the Southern Ocean caused by cooling [23] led to the emergence of vast empty ecological niches. The lack of competition allowed mutants lacking hemoglobin to leave behind offspring that populated empty habitats. The cubs developed mechanisms to compensate for mutations. Relatively isolated fjords developed habitats that were colonized by multiple individuals, resulting in six species of fish that were isolated from each other and independently lost their globin genes [22].
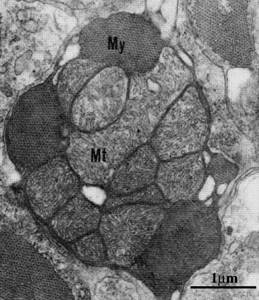
Histologically, it has been shown that a feature of ice fish is a high volume of mitochondria with a similar number and a high lipid/protein ratio in mitochondrial membranes in comparison with closely related species of the nototheniid fish family (Fig. 4). Interestingly, in white blood, which lacks myoglobin in skeletal muscle but is present in cardiac muscle, the volume of mitochondria in skeletal muscle is significantly higher than in the myocardium. Very little is known about the molecular mechanisms of this phenomenon. Presumably, this phenomenon is associated with one of the key protein regulators of mitochondrial biogenesis, PGC-1α [23].
PGC-1α is a transcriptional coactivator and central to mitochondrial formation in cells. Recently, it was discovered that PGC-1α regulates the composition and function of individual mitochondria and their oxidative metabolism. Increased oxidative metabolism is associated with increased PGC-1α activity, which is accompanied by an increase in reactive oxygen species (ROS) in mitochondria. But this protein is also a powerful regulator of ROS removal, because high levels of PGC-1α trigger the expression of numerous antioxidant enzymes [25].
[24]
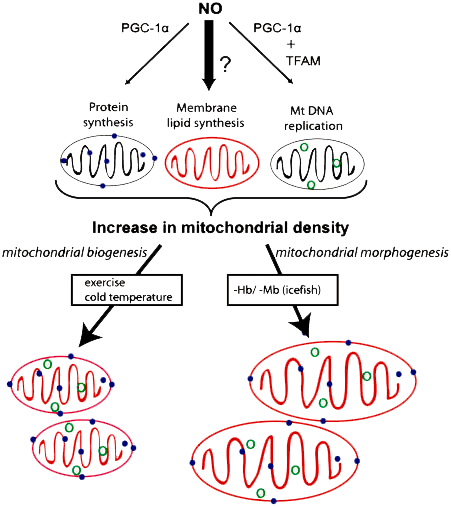
The regulator of mitochondrial membrane biogenesis in whitebloods is nitric oxide-II (NO) (Fig. 5). Compared to other fish, white bloods have an increased level of this signaling agent in the blood. In response to the loss of hemoglobin and myoglobin in the muscles of icefish, the biosynthesis of phospholipids increases, and, independently of the synthesis of mitochondrial proteins and the replication of mitochondrial DNA, this leads to an increase in the size of mitochondria. The NO molecule stimulates the formation of PGC-1α, which regulates mitochondrial DNA replication. But nothing is known about how mitochondrial phospholipid biosynthesis is integrated into this process in icefish; this may be induced by high levels of NO (dark arrow in the figure) [18].
[18]
Hemoglobin: questions of normality
What is hemoglobin? What does hemoglobin level affect?
What is hemoglobin?
Hemoglobin is part of erythrocytes or red blood cells. Hemoglobin is made up of protein and iron, which is what gives blood its red color. Hemoglobin is important for supplying oxygen to all body tissues. It also maintains blood acidity. Hemoglobin is needed not only to maintain metabolism in the body. It is an indicator of many conditions. Why are the forms of hemoglobin isolated?
- Glycated hemoglobin is a product of the addition of glucose. An increase in the level of glycated hemoglobin indicates the risk of developing diabetes mellitus. The norm of glycated hemoglobin is 4-6% of total hemoglobin.
- fetal hemoglobin - present only in the blood of children. Fetal hemoglobin levels drop after one year of life. This indicator indicates serious illnesses in adults (if it exceeds 1%).
Hemoglobin in children under one year of age decreases slightly from the day of birth. If on the first day it is up to 25 g per dl, then by 11 months it reaches 13 g/dl. And this is the norm for hemoglobin for children. Hemoglobin in women is always lower than hemoglobin in men . Hemoglobin levels between the ages of 18 and 45 are 11.7 – 15.5 and 13.2 – 17.3 g/dL, respectively.
What does hemoglobin level affect?
Hemoglobin level is a diagnostic indicator for many diseases and conditions. An increase in hemoglobin may indicate an increase in the formation of red blood cells in the blood (erythrocytosis), congenital heart defects, and intestinal obstruction. High hemoglobin is characteristic of heart failure and blood thickening. However, an increase in hemoglobin also occurs during the most ordinary events, for example, during a walk in the fresh air or after physical activity. High hemoglobin is observed in people living in the mountains or in climbers. Reduced hemoglobin leads to the development of anemia. With low hemoglobin, a person feels constant fatigue, has pale, dry skin. A decrease in hemoglobin leads to headaches, hair loss, sore eyes and poor sleep. A lack of substances involved in the production of red blood cells can cause a decrease in hemoglobin. Most often, low hemoglobin is affected by a lack of iron, vitamin B and folic acid in the body. However, low hemoglobin also becomes a sign of serious blood diseases associated with increased breakdown of red blood cells or their poor formation in the bone marrow. Hemoglobin often decreases during pregnancy, which is associated with an increased need for iron and other nutrients. At the same time, the hemoglobin norm during pregnancy corresponds to the hemoglobin norm in women. Hemoglobin is important for the development of the fetus and its oxygen supply. Therefore, throughout pregnancy, a woman should monitor her hemoglobin level. Regular walks and a varied menu help with this (liver, fish, black bread, buckwheat, almonds and eggs should be present in the diet). Physiological anemia after 30 weeks of pregnancy is associated with an increase in blood volume. Products that increase hemoglobin are the first medicine for low hemoglobin in children. After all, a lack of iron and vitamins can affect the mental and physical development of a child. With low hemoglobin, children become lethargic, their academic performance worsens, the level of physical activity decreases, and drowsiness increases. take medications to increase hemoglobin levels only as prescribed by your doctor. These include: products containing iron (maltofer, sorbifer) or products that increase the absorption of iron (ascorbic acid, folic acid).
Source:
Medstream.ru