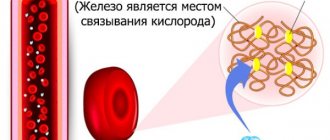
Hemoglobin is a protein that contains iron and carries oxygen from the lungs to the organs. A pathology in which the hemoglobin level falls below normal is called anemia
Anemia is accompanied by weakness, headaches, tinnitus, decreased appetite and sleep disturbances. The most vulnerable category of patients with anemia are children of early and school age. Schoolchildren get tired quickly, learn more slowly and remember less information, do not get enough sleep and become irritable. With severe anemia, dystrophic changes occur in the heart muscle - this leads to disruption of the heart and circulatory failure.
Hemoglobin levels can be raised with medications and foods. However, the choice of treatment depends on the type of anemia and its cause. In this article we will answer the questions: what is the norm for hemoglobin in children, why does it decrease, what foods does it contain, and how to prevent anemia in children.
Hemoglobin - what is the norm for a child
Each blood indicator has a norm, including hemoglobin. The World Health Organization gives the following hemoglobin standards for children:
- newborns up to 2 weeks of life - not lower than 150 g/l;
- children from 2 to 4 weeks of life - 120 g/l and above;
- children aged 6 to 59 months - 110-140 g/l;
- children aged 5 to 11 years - 115-140 g/l;
- children aged 12 to 14 years - 120-150 g/l;
- children and adults over 15 years old - 130-160 g/l.
Anything below is considered mild (below normal, but above 90 g/l), moderate (70-90 g/l) or severe (below 70 g/l) anemia. Each of the three degrees of severity has its own symptoms and treatment tactics.
Iron deficiency in adolescents
Hebiatry as it is
One of the important areas in modern pediatrics is adolescent medicine (or hebiatrics) - a branch of medicine that studies the processes of puberty, maturation, growth and development of the adolescent’s body, as well as specific diseases characteristic of this period of life.
It is obvious that the separation of adolescent medicine into an independent field is associated with the unique features of puberty - hormonal explosion and intersystem heterochrony (non-simultaneous formation of different functional systems). The development of borderline states in almost any system of the body is somehow associated with them. On the border between normality and pathology, a large group of physiological abnormalities arises, predetermined by a growth spurt and complex endocrine, vegetative and immune restructuring of the body.
It was at this time that previously hidden organic defects were added to the formation of numerous transient functional disorders: hypothalamic dysfunction with a wide range of clinical manifestations (vegetative dysfunction, metabolic syndrome, etc.), congenital inferiority of connective tissue (visceroptosis, articular hypermobility, osteochondrosis, etc.), exacerbating the already reduced adaptive abilities of the restructuring organism.2
Some of these medical problems of puberty are gradually smoothed out and overcome by the body, but others require mandatory correction. These, without a doubt, include iron deficiency - a syndrome closely related to the rapid growth process (increased volume of both muscle mass and blood) and an increase in the body's needs for iron (Fe).3 The lack of this chemical element in the body leads to a disruption in the formation of hemoglobin (Hb) and a decrease in the rate of its synthesis, the accumulation of free protoporphyrin in erythrocytes, the development of hypochromic anemia and trophic disorders in organs and tissues. Of course, this only aggravates the problems of an organism already upset by the restructuring.4
In the last century (especially in the last 50 years), significant changes have occurred in puberty - the age of its onset has decreased. Thus, the age of menarche in Central European countries and the United States decreases by 2–3 months every decade. This is due to the stability of socio-economic conditions, improving the quality of life and general health of the population.5
Deficiency of a common element
Despite the widespread occurrence of iron in nature, its deficiency in the human body is a common phenomenon. Every year, the diagnosis of “Anemia” is registered in the world (80–95% is anemia caused by iron deficiency) in more than 1.5 billion people.6-7 About a third (more than 30%) of these patients are adolescents.8
Drawing. Prevalence of iron deficiency anemia in adolescents depending on region of residence.9
And as you know, Fe is one of the main trace elements in the human body, and its importance for normal life is extremely high. Regulation of metabolism, oxygen transfer processes, tissue respiration, state of immunological resistance - all these are spheres of its influence. Iron is part of various human proteins and enzymes: about 70% of its total amount is in hemoproteins (the main representative is hemoglobin, 58% Fe); 9% – in myoglobin; up to 4% - in cytochromes, peroxidases, catalases and a number of non-heme enzymes (xanthine oxidase, NADH dehydrogenase, aconitase, transferrin, lactoferrin).
Table. The main iron-containing substrates of the body and their functions9
| Substrate | Function |
| hemoglobin (Hb) | oxygen transport |
| myoglobin | transport and storage of oxygen in muscles |
| catalase | hydrogen peroxide decomposition |
| cytochrome | tissue respiration |
| peroxidase | oxidation |
| transferrin | iron transport |
| ferritin | tissue deposition of iron |
| hemosiderin | tissue deposition of iron |
| xanthine oxidase | formation of uric acid |
| dehydrogenases | oxidation-reduction catalysis |
These endogenous costs determine the body's need for Fe. Its growth rate, nutritional status, type of nutrition, presence of chronic infection, various blood losses and other factors generate specific demand. It fluctuates over a fairly wide range. The highest is in pregnant women (5-6 mg/kg/day). It is also high in puberty – a period of rapid growth and age-related behavioral characteristics (poor nutrition due to the desire to lose weight, the philosophy of strict vegetarianism, intense sports activities, etc.) – 2 mg/kg/day. Actually, the already high demand for Fe requires the inclusion of adolescents in the risk group for the development of iron deficiency.
Normal hemoglobin concentration in adolescents:
►girls (12–18 years old) – 112–152 g/l; ►boys (12–18 years old) – 120–160 g/l.
The main causes of iron deficiency in adolescents:
- dietary restrictions;
- unbalanced diet;
- vegetarianism;
- helminthic infestations;
- anorexia;
- heavy and prolonged menstruation in girls;
- obesity (the higher the body mass index, the higher the risks);
- gastrointestinal diseases, including gastritis;
- heavy physical activity (for example, when playing sports).
Iron deficiency in this age group is noticeably more common in girls. For many authors, the explanation is obvious: the formation of menstrual function and large monthly blood losses. In Japan, according to a study, a latent form of Fe deficiency develops in 71.8% of schoolgirls already 3 years after the start of menstruation.10 Although, to be fair, these authors have opponents who believe that even heavy menstrual bleeding does not act as a major factor. causes of the syndrome, and an additional one, combined with others - with increased growth, insufficient Fe content in food.11
This condition often develops in adolescents (both boys and girls) who are overweight and obese, with iron deficiency increasing in proportion to body mass index (BMI).12-13
In some cases (especially when there is a hidden iron deficiency), the development of latent iron deficiency is provoked by sports and fitness. Intense physical activity reduces the absorption of iron in the intestine and increases its loss due to hemolysis of red blood cells, and the growth of muscle mass for the synthesis of myoglobin and hemoglobin requires more iron.
Among the risk factors, an important place is occupied by chronic diseases - pathologies of the stomach and duodenum, helminthic infestations, inflammatory bowel diseases, infectious diseases (tuberculosis, brucellosis, mycosis, etc.), atransferrinemia, collagenosis, anorexia nervosa, idiopathic pulmonary hemosiderosis, Goodpasture syndrome, etc. .9
From deficiency to anemia
Iron deficiency anemia (IDA) is a clinical and hematological syndrome characterized by impaired hemoglobin synthesis due to iron deficiency and developing against the background of various pathological (physiological) processes.
Depending on the degree of iron deficiency, three stages are distinguished:
- prelatent iron deficiency (decreased iron reserves in the depot), which has virtually no clinical manifestations;
- latent iron deficiency (decrease in the content of both deposited and transport pools of Fe); manifestations: sideropenic syndrome, various trophic disorders of the skin, its appendages and mucous membranes, perversion of taste and smell appears, astheno-vegetative syndrome develops, muscle hypotension, changes in nervous regulation, decreased immune reactivity of the body;
- iron deficiency anemia, iron deficiency anemia (complete depletion of Fe reserves, leading to a decrease in the synthesis of hemoglobin and other iron-containing compounds).
External symptoms of anemia in adolescents are manifested by anemic and sideropenic syndrome:
- dystrophic changes in the skin, hair, nail plates;
- perverted perception of tastes and smells;
- muscle pain;
- disorders of nervous regulation (expressed in a tendency to depression, mood swings, memory deterioration and academic performance);
- fatigue;
- dizziness, sometimes fainting;
- headache.
Daily iron requirement of adolescents in the Russian Federation9
| Age Gender | 11–14 years old | 14–18 years old | ||
| husband. | wives | husband. | wives | |
| Iron (mg/day) | 12 | 15 | 15 | 18 |
Classification of IDA by severity (WHO): 14-15
1. Mild anemia - the concentration of hemoglobin (Hb) in the blood is from 110 to 90 g/l. 2. Moderate anemia - Hb concentration in the blood from 89 to 70 g/l. 3. Severe anemia – Hb concentration in the blood less than 69 g/l.
Iron deficiency anemia does not threaten life and health if treatment is started on time. Otherwise, severe complications may develop.
Possible complications of iron deficiency anemia:
- heart failure;
- decreased immunity;
- exacerbation of chronic diseases;
- dysfunction of some organs.
If the Hb level critically decreases below 70 g/l, urgent hospitalization, serious treatment and a long recovery period are required. Complications in this case can be even more severe. Therefore, timely diagnosis and prevention of iron deficiency conditions is recommended for all adolescents.
Normalization and replenishment
In all cases of iron deficiency, it is necessary to establish the immediate cause of its occurrence and, if possible, eliminate it (eliminate the source of blood loss, treat the underlying disease, complicated by sideropenia). Treatment should be comprehensive and aimed not only at eliminating anemia as a symptom, but also at eliminating iron deficiency and replenishing its reserves in the body.
Since the nutritional factor is one of the main reasons for the development of iron deficiency in adolescents, it is important first of all to adjust the patient’s diet, enriching the diet with foods containing heme iron. Its best source (20–25% of iron is absorbed) is red meat (veal, beef, lamb). Chicken and pork are somewhat inferior to these types of meat. Iron from liver and fish is absorbed to an even lesser extent (it is contained in the form of ferritin and hemosiderin).
Non-heme iron, found in vegetables, fruits, and nuts, is poorly absorbed (1–5%). Moreover, many factors enhance or impair the bioavailability of Fe.
Inhibit the absorption of non-heme iron - tannins (cocoa, black tea and some types of herbal teas), carbonates and phosphates (food additives), oxalates (spinach, cabbage, beets, nuts, chocolate, tea, etc.), ethylenediaminetetraacetic acid (preservative), calcium (a lot in dairy products), plant fibers, phytic acid (walnuts, almonds, legumes, bran), drugs that reduce the acidity of gastric juice (antisecretory drugs, antacids), etc.
Enhance the absorption of non-heme iron - ascorbic, citric, succinic acids, fructose, cysteine, sorbitol, nicotinamide.
The average bioavailability of iron from the normal diet does not exceed 10%.16
But when prelatent iron deficiency turns into latent or into the highest stage - iron deficiency anemia, serious therapy is required (a balanced diet will maintain a normal Fe balance after treatment).
Two basic rules in the treatment of iron deficiency conditions: identifying and, if possible, eliminating the immediate cause of IDA and replenishing iron deficiency with iron-containing drugs (IDP).17-18
The choice of optimal iron deficiency, its dose and duration of therapeutic correction of iron deficiency in adolescents should be individual, taking into account the psychological status of the patient and all possible side effects. 19 Today the pharmaceutical market is extensive (more than 3 dozen trade names). The pancreatic acids presented on it are distinguished by their Fe content, the presence of additional components (ascorbic and succinic acids, vitamins, fructose, etc.), which affect pharmacokinetics, dosage forms and, of course, cost. Medical publications have paid a lot of attention to them in recent years: they discussed the strategy and tactics of treating patients with IDA, comparing data on the comparative effectiveness of various pancreatic acids, their bioavailability, influence on peroxidation processes, side effects, considering the clinical significance of the dosage form. These are very important professional issues that cannot be ignored.
For example, which method of drug administration should you choose? Experts who prepared national practical guidelines and WHO recommendations for the management of patients with IDA, which were based on data from a large number of clinical studies of the comparative effectiveness and tolerability of pancreas, meta-analyses and the experience of practicing doctors,20-21 give preference to oral drugs. Reason: the difference in the rate of normalization of Hb levels with oral and parenteral use of the pancreas is minimal (it all depends on the bioavailability of each specific drug), and when using, for example, Sorbifer Durules, as studies show (Carson JL and Adamson JW), in the pancreas for Intravenous administration has no advantages over oral administration in this parameter.22 No significant differences in the dynamics of the increase in Hb levels in patients with IDA who took pancreatic acid orally or parenterally were noted. And if there are no differences, then, of course, a form that is simpler and easier to use, does not cause additional stress, and increases adherence to treatment is preferable.36 And parenteral pancreatic acids are recommended as an alternative, for example, in patients with concomitant inflammatory diseases of the gastrointestinal tract.23
Another important and controversial topic concerns the choice of iron based on the valency of the iron included in the preparation - di- or trivalent (Fe2+ and Fe3+). As evidence-based medicine shows, the level of absorption of Fe2+ salts is several times higher than that of Fe3+ salts, therefore they provide a faster increase in Fe in the blood serum and Hb. Preparations containing Fe3+ require much more time to achieve the desired result (and this is if there is no copper deficiency in the body, otherwise they are ineffective), and the course of administration is longer. The degree of absorption is also reflected in the incidence of adverse effects.24
The most important requirements for oral prophylactics used in pediatric practice are good bioavailability, high safety, the availability of various dosage forms convenient for patients of all ages, as well as characteristics that ensure good adherence to treatment.
Having decided on the choice of PG based on valence – Fe2+ – we need to decide on its composition. The most common oral ferrous iron preparations prescribed to patients with IDA (children, adults, the elderly, pregnant women) continue to be ferrous sulfate (FS) based preparations. These are the first line of treatment for IDA. Their effectiveness has been confirmed by both foreign specialists25 and domestic researchers.24 Among the latter is Sechenov University professor L.I. Butler, who has studied these drugs for many years. Back in 2006, he and his colleagues studied 2 SF preparations (including Sorbifer Durules) and an iron-polymaltose complex preparation in the form of chewable tablets.26 All patients received a favorable clinical effect during therapy (significant reduction or disappearance of signs of anemia and sideropenia) and an increase or normalization of the level. The most interesting thing in this study was the magnitude and rate of increase in Hb levels. From the 2nd week, a statistically significant difference appeared between the group of patients receiving the drug SG and ZhPK (2.2 g and 1.1 g/l, respectively; p 27 As, indeed, subsequent works performed by his colleagues, for example, comparing the effectiveness 5 different preparations of iron supplements (in a daily dose of 70–200 mg of elemental iron), the magnitude and rate of increase in Hb.28 levels
These and many other studies unconditionally indicate the clinical significance of the bioavailability of one of the SF drugs - Sorbifer Durules. Even in conditions where the patient seems to be indicated for parenteral use of pancreas - after bariatric surgery (biliopancreatic bypass) for morbid obesity, i.e. in conditions of a significant decrease in the “intestinal area” of iron absorption. Long-term monitoring (5 years) demonstrated that patients who did not take PZH had significantly lower serum Hb and Fe levels starting at 4 and 3 years after surgery, respectively. That is, the bioavailability of the drug allows, in conditions of a significant reduction in the absorption area of iron, to achieve absorption adequate to maintain its homeostasis in patients of this category.29 Experts associate such a high bioavailability of this pancreas with ascorbic acid, which is part of its composition.
Sorbifer Durules contains 320 mg of ferrous sulfate (corresponding to 100 mg of ferrous iron) and 60 mg of ascorbic acid.
If we consider that the goal of pancreatic therapy is not only to normalize Hb levels, but also to replenish tissue reserves of Fe, it becomes clear that it is quite long-term. Complete normalization of Hb levels (at the maximum tolerated dose) occurs after 6–8 weeks. To replenish Fe reserves, after this, another 3 months of taking pancreatic acid are necessary (at a daily dose 2 times less than when relieving anemia). If excessive iron loss continues (during menstrual bleeding), experts recommend prescribing pancreatic iron in short courses (7-10 days monthly). And in case of relapse of anemia, a second course is indicated for 1–2 months.
Therefore, having proven the effectiveness of iron-containing preparations based on ferrous sulfate (SF), researchers did not leave aside the safety issues of their use. Based on a large clinical material (including electronic databases, Cochrane reviews, results of clinical trials - 111 clinical trials, 10,695 patients and spontaneous reports of adverse effects, NE), a comprehensive systemic analysis of the tolerability of various oral prophylactics in patients with IDA was conducted.30 Lowest incidence of NE noted when using SF containing mucoprotease (a drug with a slow release of iron): general - 4.1%, gastrointestinal dysfunction - 3.7%. And it was he who became the reference for further analysis of undesirable effects. The difference in the frequency of their development when using GF with mucoprotease and other PG is statistically significant (p
In the trade name Sorbifer Durules, the second word specifically indicates this feature of the drug. The Durules technology is based on the “packaging” of the active substance in a biologically inert plastic substance. First it is released from the surface layers; then from deeper ones. This happens gradually (over 6 hours), evenly and in small doses, so the intestinal mucosa is irritated much less and the tolerability of the drug improves, which is important during long-term therapy. After the complete release of Fe2+ ions, the empty carrier is destroyed and excreted from the intestine. By the way, this dosage form also protects against the formation of a yellow border on the teeth during long-term use.31-32
To complete the picture, a systematic review of 106 studies involving more than 10 thousand patients who were treated with various oral iron supplements was provided.33 It showed a lower incidence of gastrointestinal dysfunction with sustained-release iron supplements (3.7%) compared with other dosage forms of SF (31.6%). And it is the use of iron supplements with sustained release of iron that our foreign colleagues consider as the clinical standard for the treatment of IDA, regardless of the indications.34
Basic principles of treatment for IDA:
- therapy should be carried out primarily in the pancreas for oral administration;
- PZ are prescribed in adequate doses, which are calculated for a specific patient, taking into account his body weight and therapeutic treatment plan;
- it is preferable to prescribe pancreas containing substances that enhance iron absorption;
- simultaneous intake of food and medicinal substances that reduce iron absorption is undesirable;
- it is inappropriate to prescribe pancreatic acid if there are signs of impaired intestinal absorption;
- without special indications, it is not advisable to prescribe vitamins B1, B12, and folic acid simultaneously with pancreas;
- sufficient duration of the saturating course of pancreatic therapy (at least 1–1.5 months);
- the need to monitor the effectiveness of pancreatic therapy;
- therapy should not be stopped after normalization of hemoglobin levels;
- blood transfusions for IDA should be carried out strictly according to health indications;
- in appropriate situations, supportive therapy for the pancreas is necessary.
So, the relevance of the problem of IDA is explained by the impossibility of normal functioning of the human body, no matter how old he is, in conditions of iron deficiency. Adolescence is considered to be one of the most critical periods for this deficiency. Characteristic of puberty, serious changes in the endocrine, autonomic and immune systems, and a physiological growth spurt often lead to the formation of various functional disorders. An iron deficiency condition caused by a mismatch between the needs for this element (due to rapid growth) and its intake into the body of a teenager, without adequate correction, can lead to the development of serious complications. Therefore, IDA in patients of this category requires special attention from the doctor and emergency measures to correct iron deficiency. Moreover, modern pharmacology presents various solutions for drug therapy. This arsenal also includes an iron-containing drug that meets the most important requirements of pediatric practice - good bioavailability, high safety, availability of dosage forms convenient for adolescents, as well as characteristics that ensure good adherence to treatment - this is Sorbifer Durules. Its use provides a high average daily increase in hemoglobin levels, which makes it possible to reduce treatment time, reduce its cost and the risk of side effects, and the drug’s good tolerability and convenient dosing regimen ensure high patient compliance.
Literature
1 Shilin D.E. Isolated pubarche syndrome in girls / Guide for endocrinologists. M. 1999, p. 1–19.
2 Bokova T.A., Maslikova G.V. Iron deficiency conditions in adolescents: principles of correction. Attending doctor. No. 9, 2014, p. 49–51.
3 Maeda M., Yamamoto M., Yamauchi K. Prevalence of anemia in Japanese adolescents: 30 years' experience in screening for anemia. Int. J. Hematol. 1999. Vol. 69. Iss. 2, p. 75–80.
4 Romanova T.A. Features of the pubertal period at the present stage. RMJ. T. 12, No. 13, 2004, p. 780–786.
5 Dedov I.I., Semicheva T.V., Peterkova V.A. Sexual development of adolescent children: norm and pathology. M. 2002, p. 50–66.
6 McLean E., Cogswell M., Egli I., Wojdyla D., de Benoist B. Worldwide prevalence of anemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr., 2009, 12, p. 444–454.
7 World Health Organization. Conclusions and recommendations of the WHO consultation on prevention and control of iron deficiency in infants and young children in malaria-endemic areas. Food Nutr. Bull. 2007, 28, p. 621–627.
8 Cotta RM, Oliveira FC, Magalhães KA et al. Social and biological determinants of iron deficiency anemia. Cad. Saude Publica, 2011, vol. 27, suppl. 2, p. 309–320.
9 Botkina A.S. Iron deficiency anemia in adolescents. Pediatrician practice. November December; 2015; pp. 6–7.
10 Kagamimori S., Fujita T., Naruse Y., Kurosawa Y., Watanabe M. A longitudinal study of serum ferritin concentration during the female adolescent growth spurt. Annals of Human Biology, 1998, vol. 15, p. 413–419.
11 Idelson L.I. Hypochromic anemia. M: Medicine. 1981. 192 p.
12 Pinhas-Hamiel O., Newfield RS, Koren I. et al. Greater prevalence of iron deficiency in overweight and obese children and adolescents. Int. J. Obes. Relat. Metab. Discord. 2003, 27, p. 416–418.
13 Nead KG, Halterman JS, Kaczorowski JM et al. Overweight children and adolescents: a risk group for iron deficiency. Pediatrics. 2004, 114, p. 104–108.
14 UNICEF/UNU/WHO. Iron Deficiency Anemia: Assessment, Prevention, and Control. A Guide for Program Managers. Geneva. WHO/NHD, 2001.
15 World Health Organization et al. Haemoglobin concentrations for the diagnosis of anemia and assessment of severity. Geneva. WHO, 2011.
16 Norms of physiological needs for energy and nutrients for various groups of the population of the Russian Federation. Methodological recommendations MR 2.3.1.2432-08 (dated December 18, 2008), 41 p.
17 Kazyukova T.V., Samsygina G.A., Levina A.L. Problems of treatment of iron deficiency anemia in adolescent children. Pediatrics, 2002, No. 6, p. 4–10.
18 Gorodetsky V.V., Godulyan O.V. Iron deficiency conditions and iron deficiency anemia: treatment and diagnosis (methodological recommendations). M., 2006, 25 p.
19 Tarasova I.S., Chernov V.M. New directions in the diagnosis, treatment and prevention of iron deficiency conditions. Modern pediatrics. No. 2, 2012, p. 18–24.
20 WHO. Iron deficiency anemia; assessment, prevention and control. A guide for program managers. Geneva: World Health Organization. 2001, p. 1–114.
21 Rumyantsev A.G., Maschan A.A. Federal clinical guidelines for the diagnosis and treatment of iron deficiency anemia. M., 2015.
22 Carson JL and Adamson JW Iron Deficiency and Heart Disease: Ironclad Evidence? Hematology. 2010, vol. 4, 1, p. 348–350.
23 Stuklov N.I., Semenova E.N. Treatment of iron deficiency anemia. What is more important, effectiveness or tolerability? Is there an optimal solution? Journal of International Medicine. No. 1 (2), 2013, p. 47–55.
24 L.I. Butler. Ferrous sulfate in the treatment of iron deficiency anemia: positions retained. Therapeutic archive. No. 10, 2021, p. 108–112.
25 Auerbach M., Adamson J. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2021, 91 (1), p. 31–38.
26 Dvoretsky L.I., Zaspa E.A. Algorithms for diagnosis and treatment of iron deficiency anemia. Pharmateka. No. 5 (120), 2006, p. 117–120.
27 Dvoretsky L.I., Kolendo S.E. Sorbifer Durules in the treatment of iron deficiency anemia. International Medical Journal. No. 3–4, 1999, p. 171–173.
28 Gorodetsky V.V., Godulyan O.V., Vertkin A.L. Comparative effectiveness and tolerability of various iron-containing drugs in patients with iron deficiency anemia. Russian Medical Journal, No. 5, 2004, p. 309–315.
29 Dvoretsky L.I., Ivleva O.V. Key issues in the treatment of iron deficiency anemia. Doctor. No. 2, 2021, p. 68–73.
30 Cancelo-Hidalgo M., Castelo-Branco C., Palacios S. et al. Tolerance of different oral iron supplements: a systematic review. Curr Med Res Opin. 2013, 29 (4), p. 291–303.
31 Elwood P., Williams G. Comparative trial of slow-release and conventional iron preparations. Practitioner. 1970, 204 (224), p. 812–815.
32 McDiarmid T Johnson Diane E. Are any oral iron formulations better tolerated than ferrous sulfate? J Fam Pract. 2002, 51(6), p. 575–577.
33 Manasanch J., Castelo-Branco C., Cancelo-Hidalgo M. Tolerability of different oral iron supplements: a systematic review. In: Proceedings of the 16th WONCA European Conference; 2010.
34 Santiago P. Ferrous versus ferric oral iron formulations for the treatment of iron deficiency: a clinical overview. The Scientific World Journal. Volume 2012, Article ID 846824, 5 p. https://dx.doi.org/10.1100/2012/846824;2012:846824.
Why does a child have low hemoglobin?
Reasons for decreased hemoglobin levels in children: blood loss, insufficient blood production in the bone marrow and increased destruction of red blood cells.
Causes of low hemoglobin levels:
- malignant (cancerous) tumors;
- anemia due to vitamin deficiency;
- Iron-deficiency anemia;
- aplastic anemia;
- cirrhosis of the liver;
- Hodgkin's lymphoma;
- hypothyroidism;
- chronic kidney disease.
The first reason is blood loss. It can be acute and chronic. Typically, hemoglobin levels decrease several hours after the onset of blood loss. Chronic blood loss leads to anemia when the body does not have enough iron to produce new red blood cells. Examples of blood loss: gastrointestinal bleeding from an ulcer, bowel or kidney cancer, bone fractures, recent surgery, heavy menstruation in teenage girls.
The second reason is low blood production (erythropoiesis deficiency). In this case, a decrease in hemoglobin levels is caused by infections, hereditary pathologies, kidney diseases, copper and folic acid deficiency, vitamin B1 deficiency and malabsorption.
In addition, insufficiency of erythropoiesis occurs in myelofibrosis and osteomyelosclerosis. Myelofibrosis is a pathology in which fibrous tissue gradually replaces hematopoietic tissue in the bone marrow. Osteomyelosclerosis is the replacement of connective tissue with hematopoietic tissue. These disorders usually occur in cancers: lymphoma, multiple myeloma, chronic myelogenous leukemia.
The third reason is increased destruction of red blood cells. Occurs with pathological enlargement of the spleen, taking certain medications (quinine, quinidine, penicillin, ticlopidine), hemolytic-uremic syndrome, Epstein-Barr viral infection (human herpes virus type 4), malaria, botulism, tetanus, congenital heart valve defects, spider bites , insufficient levels of phosphates in the blood.
Other reasons for low hemoglobin levels in the blood:
- inflammatory diseases of the small and large intestine;
- insufficient intake of iron into the child’s body;
- chronic kidney disease;
- prematurity of the child;
- late or early umbilical cord ligation;
- Giardiasis is a parasitic intestinal disease;
- Helicobacter pylori infection and atrophic gastritis.
There are groups and risk factors that can lead to low hemoglobin levels and the most common type of anemia, iron deficiency:
- if the child was born with low body weight;
- children born from multiple pregnancies;
- children who did not receive enough iron through breastfeeding and formula milk;
- children who often suffer from infectious diseases;
- children with allergies;
- malnutrition, poverty.
A decrease in hemoglobin levels is not always regarded as a pathology. Children, especially teenagers, need a lot of iron for their body development. Due to the rapid growth of the microelement, there may be a temporary lack of microelement, so the level of hemoglobin in the blood decreases. This is especially true for children and adolescents who are actively involved in sports. They may develop "athlete's anemia." By eating foods high in iron, the deficiency is eliminated.
Types of disease
Experts distinguish several types of anemia:
- acute, occurring after an injury with significant blood loss or frequent nosebleeds;
- hemolytic, which occurs in cases of destruction of hemoglobin-containing red blood cells before performing their functions;
- symptomatic, signaling the presence of chronic diseases in the child’s body;
- iron deficiency, which is the most common.
Treatment of low hemoglobin in a child
The choice of treatment method for hemoglobin in a child depends entirely on the causes of the disease and on individual indicators. After determining ways to eliminate the causes of the disease, the pediatrician gives parents recommendations regarding the baby’s diet and daily routine. The doctor will tell you about contraindications, preventive measures, etc. Effective measures include iron replenishment therapy.
How does low hemoglobin manifest?
Signs of low hemoglobin levels manifest themselves in different ways. They can be divided into groups of symptoms: asthenic, epithelial, cardiovascular, muscular and secondary immunodeficiency syndrome.
Asthenic symptoms are manifested by increased fatigue, irritability, emotional instability, lethargy, and absent-mindedness. Children sleep poorly, eat little or do not want to eat at all. Children may complain of tinnitus, dizziness and headaches.
Epithelial symptoms: children have a pale face, areas of the legs and nails, pale ears and oral mucosa. The skin is dry and often flakes. Hair becomes brittle and may fall out. “Sticks” appear in the corners of the mouth. The child may complain of a burning tongue and dry mouth, difficulty swallowing and nausea.
Cardiovascular symptoms: increased heart rate, rarely shortness of breath. Sometimes the child complains of pain in the heart area.
Muscle symptoms: muscles weaken, the child quickly gets tired of simple activities, and may involuntarily defecate day and night due to weakness of the sphincter muscle.
Secondary immunodeficiency syndrome is manifested by the fact that the child often suffers from colds. He often experiences otitis media, pneumonia and intestinal infections. Rare symptoms of low hemoglobin levels are an increase in body temperature to 38 0C, red-tinged urine, swelling on the face.
Prognosis and prevention
Iron replacement therapy is an effective method for treating iron deficiency anemia (IDA) in children. The correct selection of medication and dosage will help you quickly cope with the disease. In the absence of timely therapy, the prognosis can be unfavorable; there is a high risk of developing disorders associated with oxygen starvation of the child’s body tissues. Therefore, you should consult a pediatrician when the first alarming signs appear, and be observed by a doctor for preventive purposes after suffering severe infections, injuries and interventions.
Specialists do not only analyze symptoms and treat anemia in children. You can contact a pediatrician or hematologist for preventive purposes: regular examinations are of great importance. It is important to know that nutritional deficiencies can be detected early enough to prevent the development of unpleasant symptoms and complications. Children born prematurely and/or with underweight are at risk for developing this syndrome. It is also important to monitor the condition after injuries, operations, and severe infectious diseases.
You can reduce the likelihood of developing anemia with the help of simple recommendations: monitoring the child’s diet, regular walks and a strict daily routine, moderate physical activity.
Low hemoglobin - what to do
If you notice any of the symptoms described above in your child, contact your pediatrician. He will examine the child, listen to heart sounds, and give a referral for a general and biochemical blood test, which can confirm a reduced hemoglobin level. After the doctor has diagnosed anemia and identified its cause, the child is prescribed treatment and diet.
Depending on the cause of anemia, the doctor prescribes different treatments. For example, in case of deficiency anemia (when the body does not receive enough nutrients), medications are prescribed to compensate for the deficiency: B vitamins or iron supplements. For B12-deficiency anemia, the doctor prescribes vitamin B12 (cyanocobalamin), and in severe cases, a transfusion of red blood cells is indicated. For anemia of a chronic disease, iron supplements and recombinant erythropoietin are prescribed.
Often, anemic syndrome manifests itself in other diseases, for example, with kidney pathology, infections or disorders of the gastrointestinal tract. In this case, the doctor prescribes treatment for the underlying disease, which will restore the level of hemoglobin in the blood.
How to make an appointment with a pediatrician
The pediatric department, created on the basis of JSC "Medicine" (clinic of academician Roitberg) in Moscow, offers European-level services for the diagnosis, treatment and prevention of low hemoglobin. To receive highly qualified medical care, you can make an appointment with a pediatrician who will conduct an examination and comprehensive medical examination of the child.
To make an appointment with a specialist at the Pediatric Department of JSC “Medicine” (clinic of Academician Roitberg), use one of the following methods:
- quick registration form on the website;
- by telephone 24/7;
- using a mobile application.
JSC "Medicine" (clinic of academician Roitberg) is located in the center of Moscow at 2nd Tverskoy-Yamskaya lane, 10, a five-minute walk from the Mayakovskaya metro station.
Foods that increase hemoglobin
Food can increase hemoglobin levels in iron deficiency anemia. For other types (dyshematopoietic, posthemorrhagic, hemolytic, B12-deficiency anemia), specific treatment with drugs, blood transfusions or surgery is required.
List of products that increase hemoglobin:
- tahini halva contains more than 50 mg of iron per 100 g of product. Halva also contains vitamins E, B, phosphorus, zinc and calcium. Some of these substances improve the absorption of iron in the digestive tract;
- meat products: beef, liver, tongue, rabbit, veal. The iron content in these products ranges from 5 to 30 mg per 100 g of product;
- dried mushrooms: they contain up to 30 mg of iron per 100 g of finished product;
- seafood: squid, shrimp, clams, caviar, scallops. They contain up to 30 mg of iron per 100 g of product;
- wheat bran contains up to 15 mg of iron per 100 g of product;
- seaweed contains up to 12 mg of iron per 100 g of product;
- beets - 30 mg of microelement per 100 g of product;
- pomegranate - up to 30 mg of iron per 100 g of product.
It is recommended to add foods enriched with cobalt and manganese to your daily diet: squid, tuna, cod, catfish, flounder, pike, oat bran, rice flour. These products help the absorption of iron and accelerate the production of hemoglobin. It is also recommended to add medicinal herbs to food: stinging nettle, tripartite string, infusion of rose hips, strawberries, rowan tea, black currants.
Methods for diagnosing anemia in children
The main research method if you suspect that a child has anemia is laboratory tests
. Thus, in a clinical blood test, a decrease in hemoglobin levels is immediately visible - less than 110 g/l, as well as Er, serum iron CP, ferritin concentration, vitamin content, bilirubin, transferrin saturation with iron.
a bone marrow biopsy is needed to determine the exact cause of symptoms.
followed by histological examination.
Diagnostics allows you to determine the severity and form of anemia. Based on its results, the child may require consultation with highly specialized doctors (nephrologist, gastroenterologist, gynecologist, etc.), examination of the kidneys (ultrasound) and gastrointestinal tract organs (ultrasound of the abdominal cavity, endoscopy).
Treatment of anemia in children
When anemia in children is not an independent disease, but a symptom of another pathology, all measures are aimed at treating the primary focus. In other cases, the first thing parents should do if a child has anemia is to adjust his diet. Not only a balanced diet is necessary, but also a proper eating regimen.
Older children are recommended to consume more beef, liver, seafood, greens, legumes, green vegetables and fruits, vegetable and fruit juices (freshly squeezed, not packaged).
If an infant who is being fed breast milk suffers from anemia, then the mother’s diet must first be adjusted - include not only iron-rich foods, but also iron supplements and multivitamin complexes. You should not delay the introduction of complementary foods - meat puree, egg yolk, fruit and vegetable juices, vegetables. When a child is bottle-fed, the pediatrician must prescribe a special milk formula with a high iron content.
Clinical recommendations for children with anemia also include additional sleep, sufficient walks in the fresh air, ultraviolet radiation, massage, and daily exercises.
For anemia, drug treatment is also carried out, including iron supplements, multivitamin complexes for an average period of 1.5-2.5 months or until clinical blood parameters in children are normalized. If the case is severe, the doctor may prescribe a blood transfusion (transfusion of red blood cells). Source: M.V. Erman Iron deficiency anemia in children // Health is the basis of human potential: problems and ways to solve them, 2013
Complications of the disease
If anemia exists in a child for a long time, its consequences may be: hair loss, highly brittle nails. A severe form of the pathology can lead to increased bleeding (hemorrhagic syndrome) and loss of consciousness.