Diphtheria
(diphtheria infection) is an acute infectious, bacterial disease. The causative agent is a rod-shaped bacterium of the species Corynebacterium, which is also called diphtheria bacillus or Loeffler's bacillus after its discoverer.
The disease is transmitted by airborne droplets and household contact. It mainly affects the oropharynx, slightly less frequently the nose, and upper respiratory tract. May affect eyes, skin and genitals. In Russia, vaccination against diphtheria is carried out starting from 3 months of age, so cases of the disease are quite rare. According to WHO, 86% of children worldwide receive three recommended doses, resulting in stable, lifelong immunity.
Causes
The source of the disease is an infected person: a patient or a carrier of a pathogenic strain. The pathogen is released from the body during the recovery period for quite a long time: from 15 days to 3 months. The route of transmission is aerosol, most often infection occurs by airborne droplets, less often by household contact or airborne dust. The stick can remain active on dishes and towels used by the patient. Able to multiply in food products: ready-made meals, meat, milk, cheese.
The bacterium is resistant to the external environment and remains viable for 2 months. It does not linger long on smooth surfaces; it can be found on soft toys, clothes, bedding, carpets, and wool products. The diphtheria bacillus tolerates cold, but dies at a temperature of +60 C within 10 minutes. Ultraviolet radiation (UV lamps and direct sunlight), disinfectants containing Lysol or chlorine are also harmful to it.
Classification
Diphtheria is divided into localized and widespread forms.
According to the options, the flow is divided into 4 forms:
Diphtheria of the oropharynx, which is divided into:
- Localized, characterized by catarrhal, island and film inflammation of the palatine tonsils.
- Common, distinguishable by the presence of plaque not only on the tonsils, but also on other mucous membranes of the oropharynx.
- Subtoxic, hypertoxic and toxic from 1 to 3 degrees.
- Diphtheria croup of the larynx, a form with several variants of the course:
- Localized (only the larynx is affected).
- Common, in which the trachea is also affected.
- Descending, also affecting the bronchi.
- Diphtheria localized on other organs (parts of the body): diphtheria of the nose, eyes, skin, genitals.
- A combined form of the disease, in which several organs are affected in a combination not listed above.
In the international classification of diseases, diphtheria has code A36.0 and several forms, based on localization: diphtheria of the pharynx, nasopharynx, larynx, skin, other and unspecified.
Diphtheria symptoms
Classic symptoms, identical to most forms of diphtheria, include:
- temperature increase;
- severe weakness;
- not severe pain in the throat;
- difficulty swallowing;
- tightly sealed plaque on the tonsils, often gray-white in color;
- swelling of the neck and mucous membranes, enlargement of the tonsils and cervical lymph nodes.
In some forms of the disease, symptoms may differ. In 75% of cases, a localized form is diagnosed. In 90% of cases, nasopharyngeal diphtheria is diagnosed. The onset is acute with moderate intoxication of the body, accompanied by an increase in body temperature to +39 C. Fever lasts no more than 3 days. Then a plaque appears, thickens and becomes shiny. Any attempts to remove it cause bleeding.
Oropharyngeal diphtheria
The localized form is expressed in the appearance of fibrinous (more often) or loose (less often) on the palatine tonsils. The nasopharynx becomes inflamed, the lymph nodes enlarge unevenly. Inflammations can be bilateral or unilateral. The temperature, as a rule, does not rise, intoxication is mild. The pain is minor.
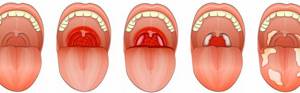
Localized diphtheria of the oropharynx is one of the mildest forms. With timely treatment, it does not progress and quickly ends in recovery. If the disease was accompanied by a fever, it goes away within 2–3 days, and the plaque on the tonsils disappears after a week.
Common
The form is rare and is distinguished by the presence of plaque not only on the tonsils, but also on the surrounding mucous membranes. Pain syndrome, intoxication, enlarged lymph nodes are more severe. There is pain in the throat and neck area, and the patient has difficulty swallowing. The temperature is elevated. Of the main symptoms:
- Purple color of tonsils.
- Plaque on the tonsils, uvula and palatine arches.
- Painful swelling of the subcutaneous tissue above the lymph nodes. The inflammation is often unilateral.
Toxic form
more often diagnosed in adults. It is characterized by a violent onset with temperatures rising to high values. Among the main symptoms:
- Tachycardia.
- Cyanosis of lips.
- Arterial hypotension.
- Intense pain in the throat, neck, abdomen.
Against the background of intoxication and high temperature, nausea, vomiting, delirium, and agitation are possible.
The hypertoxic form of diphtheria infection is the most severe. It develops against a background of weakened immunity, in people with chronic diseases. For example, alcoholism, cirrhosis, AIDS, diabetes. The disease is dangerous with a high probability of developing hemorrhagic syndrome with adrenal insufficiency. Without timely medical care, a person can die within 1 - 2 days.
Diphtheria croup
- Localized form
. Pathological processes are organic to the laryngeal mucosa.
- Common form
. The larynx and trachea are infected.
- Descending form
. In addition to the larynx and trachea, the bronchi are also affected.
The disease is more common in children and infants. In recent years, the number of infections among adults has been increasing. Sometimes croup accompanies oropharyngeal diphtheria; in particularly severe forms, pathological processes can extend from the oral cavity to the bronchi. At the same time, intoxication is mild, nausea and fever may occur. The infectious process is accompanied by swelling of the mucous membranes, inflammation of the respiratory tract and vocal folds of the larynx.
The disease goes through three successive stages:
- Dysphonic
- hoarseness of voice, “barking” cough, noisy breathing, pale skin. The duration of the stage is from 1 to 7 days.
- Stenotic stage
(larynx stenosis) - narrowing of the lumen leading to difficulty breathing.
- Asphyxial
- the narrowing of the larynx reaches a peak, sometimes it is completely blocked, leading to obstruction of the airways. Before this stage occurs, the patient must undergo treatment in a hospital. Otherwise, hypoxia will lead to disruption of brain function, and a person may die from suffocation.
Nasal diphtheria
It is expressed in the presence of fibrinous or filmy plaque on the mucous membrane. The discharge is serous-purulent, breathing through the nose is difficult. The skin around the nostrils is irritated. Body temperature rarely rises, there are no signs of intoxication. Nasal diphtheria often accompanies lesions of the oral cavity.
Diphtheria eye
Conjunctivitis, often unilateral, is characterized by a moderate serous discharge. Inflammation of the conjunctiva is accompanied by swelling of the eyelids. Intoxication is mild, the temperature does not rise.
Toxic diphtheria of the eyes is characterized by severe swelling of the eyelids, pronounced purulent discharge, and an acute onset. Almost always affects both eyes. General intoxication of the body and fever are noted.
Other forms of diphtheria
This includes diphtheria infections of the ear, skin, and genitals. They are rarely diagnosed and are associated with the method of infection. May accompany diphtheria of the oropharynx or nose. The affected areas are swollen, the nearest lymph nodes are inflamed. Fibrinous deposits and purulent discharge are observed.
Skin infections develop in places of damage: scratches, wounds. Diphtheria of the genital organs causes lesions of the foreskin in men and the vaginal mucosa in women. Nearby tissues and mucous membranes may be affected. Urinating with an inflamed urethra causes pain, and there is purulent discharge. The general condition of the patients is satisfactory, without symptoms of intoxication and fever. Unlike other forms, the disease can proceed slowly and for a long time.
Asymptomatic carriage
In persons who have previously had diphtheria, asymptomatic carriage can be observed. The transition of the disease to this stage is noted in people with chronic diseases, in particular with chronic inflammation of the nasopharynx. Carriage periods vary from person to person.

Technique for taking nasal and throat swabs for Loeffler's bacillus (on BL)
Purpose: To establish the nature of the bacteriological sphere, determine its sensitivity to antibacterial drugs.
Indications: Doctor's orders
Conditions for the procedure : It is carried out in the morning before eating and rinsing the mouth and pharynx with disinfectants. In urgent cases, during the day, but within 2 hours after eating.
Equipment:
- 2 sterile dry test tubes, labeled “N” (nose), “Z” (throat), inside which is a cotton swab wound on a wire passed through a stopper, closed with a cotton plug
- tripod, sterile spatula, gloves, mask, referral form
Possible problems: Child's anxiety, refusal of manipulation.
Stages:
1. Preparation for manipulation:
1.1 Prepare everything you need
1.2 Wash your hands, put on gloves and a mask
2. Performing the manipulation:
2.1 Place the child facing the light; the assistant fixes the child’s legs with his feet, his left hand with his hand, and his head with his right hand, placing his palm on the child’s forehead and slightly tilting his head.
2.2 Take one of the test tubes in your left hand and slightly lift the tip of the child’s nose with your thumb.
2.3 With your right hand, remove the swab from test tube “H” without touching the walls and edges of the test tube.
2.4 Insert the tampon, without touching the outer surface of the nose, first into one, then into the other nasal passage and remove the mucus.
2.5 Carefully, without touching the edges of the outer surface and walls of the test tube, insert the swab inside it, place the test tube in a stand.
2.6 With the same precautions, remove the swab from the 2nd test tube “Z”.
2.7 Take the spatula in your left hand and open the child’s mouth, press on the root of the tongue.
2.8 Carefully, without touching the mucous membrane of the oral cavity and tongue with the swab, remove plaque from the right tonsil, right palatine arch, small uvula, left arch, left tonsil and posterior pharyngeal wall.
2.9 Place the swab back into the tube using the same precautions.
2.10 Attach the direction to the laboratory to the upper end of the wire.
2.11 To avoid drying out, immediately send the collected material to the laboratory.
End of the procedure:
3.1 Remove gloves, immerse in disinfectant solution
3.2 Wash and dry your hands, remove the mask
Note:
1. The material must be delivered to the laboratory no later than 3 hours.
2. If the laboratory is remote, place the material in a transport medium.
Technique for administering the hepatitis B vaccine.
Goal: active immunization of the child, prevention of hepatitis B disease.
Indications: doctor's prescription.
Contraindications: hypersensitivity to vaccine components, acute infectious and non-infectious diseases, pregnancy.
Equipment: running water, soap, towel, gloves, vaccine, disposable syringes, needles, sterile material, sterile tweezers, alcohol, container with disinfectant solution, tray.
Safety precautions: strict adherence to aseptic rules, work with gloves, do not leave the child unattended.
Possible problems: child’s anxiety, fear of the procedure, negative attitude of parents towards vaccination, likelihood of complications.
Note! Before vaccination, the child must be examined by a pediatrician and body temperature measured.
Stages:
1. Preparation for manipulation:
1.1 Examine the child.
1.2 Wash your hands, put on gloves.
1.3 Check the ampoule or bottle of vaccination material for integrity, expiration date, number of vaccine doses.
1.4 Shake the vial or ampoule with the vaccine until a uniform suspension is obtained.
2. Performing the manipulation:
2.1 Open the ampoule and take the vaccination dose of the vaccine (0.5 ml) from the ampoule or vial, observing all the rules of asepsis
2.2 Treat the injection site with 70% alcohol.
2.3 Inject the vaccine intramuscularly into the deltoid muscle for older children, and into the anterolateral thigh for newborns and young children.
2.4 Remove the needle and treat the injection site with 70% alcohol.
2.5 Dip the used syringe and cotton swabs into the disinfectant solution.
3. End of manipulation:
3.1 Wash your hands, discard gloves in a disinfectant solution.
3.2 Enter vaccination data into the preventive vaccination card (063/u) and into the child’s development history (f. 112/u) indicating the date of vaccination, dose, number, series, manufacturer, reaction to vaccination.
3.3 Observe the child after vaccination for 30 minutes.
Note: Vaccination reaction: in 5-10% of cases, pain, erythema and induration may occur at the injection site.
For vaccination, a recombinant yeast vaccine against hepatitis B, produced domestically (Combetex LTD) or imported (Engerix B), is used.
The vaccine is available in ampoules or vials of 0.5 or 1 ml.
The vaccination course is carried out according to the standard scheme:
1 dose – in the first 12 hours of life.
2nd dose – after 1 month.
3rd dose – 6 months after the first vaccination.
Technique for irrigating the oral cavity and pharynx in children under 3-4 years of age.
Goal: reduce inflammation.
Indications: doctor's prescription.
Equipment: running water, soap, towel, mask, gloves, boiled rubber cylinder, irrigation solution, tray or basin, spatula, container with disinfectant solution.
Safety precautions: do not leave the child unattended.
Possible problems: child anxiety, feeling of fear.
Stages:
1. Preparation for manipulation:
1.1 Explain to mom the meaning of the manipulation.
1.2 Wash your hands, put on gloves and a mask.
1.3 Place the child in the arms of an assistant, who secures his arms and head.
2. Performing the manipulation:
2.1 Fill a rubber container with medicine and prepare a tray or basin.
2.2 Take the spatula in your left hand and open the child’s mouth.
2.3 With your right hand, bring the can to the child’s mouth and direct the stream of liquid to the hard palate, while the assistant tilts the child’s head down, first on one side, then on the other, the water from the mouth flows into the tray (basin) provided.
3. End of manipulation:
3.1 Dip the used rubber can into the disinfectant solution.
3.2 Remove gloves and place them in a disinfectant solution.
3.3 Remove the mask.
Note: only children over 3-4 years old can rinse their mouths and pharynx on their own. It is important to teach the child to hold liquid so that it falls on the back wall of the throat.
Technique for vaccination against tuberculosis.
Goal : Creation of immunity against tuberculosis.
Indications : Negative Mantoux test no earlier than 3 days and no later than 2 weeks after the Mantoux test.
Contraindications: Positive Mantoux test.
Equipment:
— running warm water, soap, towel, gloves
- tuberculin syringe, 70% alcohol, vaccine, solvent
- sterile tray, waste material tray
- container with disinfectant solution, cotton wool
Safety precautions: monitoring the child after vaccination.
Vaccination is carried out for all healthy children on days 3-7 strictly intravenously at the border of the upper and middle third of the outer surface of the left shoulder.
I Revaccination at 7 years of age for tuberculin-negative children.
II Revaccination at 14 years of age for teberculin-negative children.
BCG vaccine – live bacteria of the BCG vaccine strain, which is 20 doses. To dilute the vaccine, 2 ml of sterile saline solution is required.
Stages:
1. Preparation for manipulation:
1.1 Explain to the parents (patient) the meaning and course of the manipulation
1.2 Wash your hands, wear gloves
2. Performing the manipulation:
2.1 Take a 1 ml ampoule with the vaccine and dissolve it in 2 ml of saline solution (20 doses)
2.2 Take the required dose
2.3 Treat the injection site with alcohol, making strokes in one direction
2.4 Pull the skin at the injection site with your left hand
2.5 Insert the tip of the needle into the skin, holding it with the bevel upward almost parallel to the skin
2.6 Fixing the needle on the skin, press it against the skin with the 2nd finger of your left hand
2.7 Place your left hand on the piston, inject the drug, and a papule will form on the skin
2.8 Remove the needle without pressing the injection site with a cotton ball containing alcohol
3. End of manipulation:
3.1 Immerse the material used in the disinfectant solution
3.2 Remove gloves and dip them in disinfectant solution
3.3 Wash your hands
Nature of the vaccination reaction: A white papule forms at the site of vaccine administration, which will disappear in 15-20 minutes, after 4-6 weeks a papule 2-10 mm in size will appear, then a pustule, a crust, and at 6 months a scar.
Complications are rare. Cold abscesses occur during subcutaneous injection of the vaccine. Post-vaccination lymphadenitis.
Note: The vaccine can only be used for 2 hours after opening the ampoule, and then it is inactivated.
Diagnosis of diphtheria
Often, a clinical diagnosis of diphtheria is made without laboratory tests based on the presence of gray films on the mucous membrane of the throat. This can occur in small towns, with a mild course of the disease in relatively healthy adults. When examining children, people with chronic diseases, or when the clinical picture is unclear, laboratory testing is recommended.
The most commonly performed laboratory tests are:
- bacteriological smear analysis;
- serological analysis;
- PCR analysis;
- clinical blood test;
- antibody test.
Diphtheria croup is diagnosed by examining the larynx through a laryngoscope. The development of neurological pathologies requires the supervision of a neurologist. If there are signs of diphtheria myocarditis, a cardiologist will perform an ultrasound of the heart and an ECG.
Brief description of laboratory tests for diphtheria
Bacteriological analysis
The smear is fundamental for making a diagnosis. For research, biomaterial is taken and placed in a nutrient medium. It remains in the laboratory for 5–7 days, during which time it is possible to detect not only the development of the diphtheria bacillus, but also the presence of its toxigenic properties. If a toxigenic strain is identified, it is necessary to examine all those in contact with the patient.
, PCR diagnostics can be performed
. It is not required for diagnosis. The material is collected according to a similar scheme. PCR allows you to identify the pathogen and determine whether it is toxic.
Antibody tests
(RPGA or ELISA) are used to make a diagnosis. They take less time than bacteriological smear analysis, from 2 to 5 days. They allow you to assess the level of immunity (the degree of protection of the body from infection). Therefore, these tests are prescribed to people before employment in medical institutions and other structures that are at risk. Venous blood is required for the study.
Regardless of the diagnostic method of RPHA or ELISA, the test results are interpreted in the same way. The level marker is 1:10 or 0.01 IU/ml. If the value obtained is lower, then the chance of getting sick is high and vaccination is necessary.

Clinical blood test
is an auxiliary method, since it allows only to detect the presence of bacterial inflammation. The result cannot be used for differential diagnosis; it is impossible to distinguish diphtheria from tonsillitis. To perform CAO, venous blood is used.
Once the pathogen is identified or if diphtheria is suspected, the clinician must notify health authorities within 24 hours. This is necessary to organize the administration of a dose of antitoxic diphtheria serum (APDS) to an infected person, as well as to conduct an epidemiological investigation and identify the circle of contact persons. The health service is given 48 hours for this event.
Material collection
Proper collection of material for research includes separate swabs from the throat and nose.
Each smear is collected with a special thin loop of wire wrapped in sterile cotton wool. To collect material from the nose, a loop is inserted into the patient first into one nostril and then into the other, approximately 10 - 20 mm, and carefully passed along the mucous membrane of the nasal passages.
To identify the diphtheria bacillus, a loop with the material for analysis is immersed in a dry sterile tube and delivered to the laboratory within 3 hours.
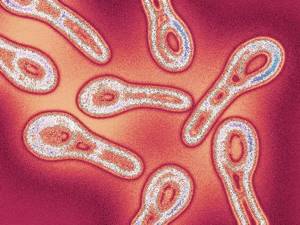
If transportation to the laboratory takes longer, then the loops with the material for analysis are immersed in a glycerin solution for preservation.
Before the procedure, the patient is recommended to clean the nasal cavity (blow his nose) without the use of additional products, solutions or medications.
READ Should you be afraid of mobiluncus in a smear?
To take a smear for BL from the throat, the doctor will need a special wire loop with sterile cotton wool and a sterile spatula (most often disposable).
The doctor presses the root of the patient’s tongue with a spatula so that the collection loop does not touch the oral mucosa, especially the tongue.
Then carefully passes a loop of cotton wool along the palatine arches, tonsils and the posterior wall of the oropharynx.
- Analysis for staphylococcus. How to take a throat swab for staphylococcus?
The most reliable is considered to be a smear taken from the border of inflamed and healthy tissues, near the foci of plaque - it is in these areas that the maximum number of pathogenic bacteria accumulates.
Just like the material for a smear from the nasal cavity, the spatula with the resulting material is placed in a dry sterile test tube or glycerin solution.

Before the procedure, the patient is recommended to avoid the use of antimicrobial or antibacterial drugs (lozenges, inhalers, solutions, herbal decoctions, etc.)
At least 2 hours before sampling, you must refrain from eating food and liquids, do not brush your teeth or gargle.
Treatment
Diphtheria is treated by infectious disease specialists; in case of a complicated course of the disease, other specialists may be involved. Patients are hospitalized in infectious diseases departments of clinical hospitals. Outpatient treatment is unacceptable, even for asymptomatic carriers.
Therapy is based on the administration of anti-diphtheria antitoxic serum. Additional medications include:
- If a secondary infection develops, antibiotics are prescribed.
- For toxic forms of diphtheria, glucose, cocarboxylase, and glucocorticoid drugs are prescribed. If necessary, plasmapheresis (filtration of blood plasma) may be prescribed to remove toxic substances.
- If there is a threat of asphyxia, tracheal intubation is performed (insertion of an endotracheal tube). In case of obstruction by films that violate the obstruction of the upper respiratory tract, a tracheostomy is performed (the creation of a cavity in the trachea by surgical means).
Complications
Diphtheria poses a serious danger due to its complications, both during the disease and after recovery. Most often, the course of the disease is complicated by toxic nephrosis (acute kidney damage), adrenal insufficiency, and infectious-toxic shock leading to coma. Inflammation of myocarditis and the trunks of the nervous system is common. Among the serious complications is paralysis of the respiratory tract, which can lead to asphyxia.
The most common cause of death is myocarditis, which usually occurs at the end of the first, beginning of the second week from the moment of infection. Damage to the nervous system can occur both during the period of illness and several months after recovery. Early and late peripheral paralysis are also observed. This leads to the following consequences: nasal voice, choking while eating, throwing it out through the nose, paralysis of the facial muscles, squint, ptosis, inability to distinguish small objects close up.
Diphtheria
Diphtheria is caused by toxigenic strains of Corynebacterium diphtheriae or Loeffler's bacillus; non-toxigenic strains do not cause the disease. In terms of its properties, diphtheria toxin is a potent poison, second only to botulinum and tetanus. Under the influence of the toxin, the synthesis of proteins in the internal organs of the patient is disrupted, which leads to structural and functional disorders, demyelination of nerve fibers - to paralysis and paresis. Only lysogenic strains of C.diphtheriae infected with a bacteriophage (p-phage) carrying the tox gene, encoding the structure of the diphtheria toxin, exhibit the ability to form toxins. The transfer of the tox gene by the bacteriophage to nontoxigenic strains of C.diphtheriae living in the nasopharynx and their accumulation in the population may be accompanied by the development of a diphtheria outbreak.
The closest taxonomically to the species C.diphtheriae are C.ulcerans and C.рseudotuberculosis, natural pathogens of large and small livestock and horses. In addition, Hoffmann's bacillus (C.pseudodiphtheriticum), which is not pathogenic or toxigenic for humans, is often found on the mucous membrane of the oropharynx and nose. Therefore, the main task of laboratory diagnosis of diphtheria is to identify toxigenic strains of diphtheria and differentiate the causative agent of diphtheria from other corynebacteria, normal inhabitants of the nasopharynx and oropharynx. All microorganisms of the genus Corynebacterium are gram-positive polymorphic rods that do not form spores and grow well under aerobic conditions at 37°C. When stained with methylene blue, intracellular striations are observed in C.diphtheriae cells, which is explained by the presence of volutin grains. Gram staining is not used due to variability in cell staining. Based on the shape of the colonies and some biochemical properties, C.diphtheriae are divided into cultural and biochemical variants - gravis, mitis, intermedius, but they all have the ability to produce diphtheria toxin. The severity of the disease is not associated with the biochemical variant of C.diphtheriae. Toxigenic strains of diphtheria are more sensitive to antibiotics than non-toxigenic strains.
Indications for examination
- Preventive examination – identification of sources of infection, population groups with an increased risk of disease; monitoring the circulation of toxigenic strains in the population; persons entering orphanages, boarding schools, specialized institutions for children and adults;
- examinations for epidemic indications - persons with confirmed contact with a patient with diphtheria or during a diphtheria epidemic in a given area;
- diagnostic studies - if diphtheria is suspected (tonsillitis, nasopharyngitis or laryngitis that occurs with pseudomembranous plaques).
Differential diagnosis.
Diseases accompanied by tonsillitis (infectious mononucleosis, streptococcal tonsillitis, staphylococcal etiology, etc.).
During cultural studies, the pathogen C.diphtheriae is differentiated from other corynebacteria, primarily C.pseudodiphtheriticum, C.ulcerans, C.pseudotuberculosis.
Material for research
- A smear from the oropharynx and nose, if diphtheria is suspected in rare locations (eye, wound, ear, etc.) - material from the affected areas, as well as from the tonsils and nose - cultural studies, detection of a specific fragment of the tox gene;
- blood serum – detection of AT.
Etiological laboratory diagnostics include inoculation of clinical material with the study of toxigenic properties on a medium to determine toxigenicity and biochemical identification of the pathogen, detection of antigen (diphtheria toxin), identification of specific antibodies to diphtheria toxin, detection of a specific fragment of the tox gene.
Comparative characteristics of laboratory diagnostic methods and features of interpretation of their results.
Microscopic examinations are performed only to identify the isolated culture. Microscopy of biological material is not performed.
For sowing, material from appropriate localizations is used. The study of toxigenic properties on a medium to determine toxigenicity is performed using the method of counter immunodiffusion of toxin and antitoxic antibodies in a solid nutrient medium. If there are no precipitation lines on the medium for determining toxigenicity, after 48 hours of incubation the culture is considered non-toxicogenic. For biochemical identification, a cystinase test (Pizou medium) and determination of urease and saccharolytic (sucrose, glucose, starch) activity are used.
Issuing a response:
- when specific lines of precipitation are detected on the medium to determine toxigenicity after 24–48 hours, a positive test for cystinase, a negative test for urease, characteristic cultural and biochemical properties, a conclusion is given about the isolation of a toxigenic strain of C.diphtheriae belonging to the cultural-biochemical variant gravis, mitis;
- in the absence of specific precipitation lines on the medium to determine toxigenicity after 48 hours, a positive test for cystinase, a negative test for urease, characteristic cultural and biochemical properties, a conclusion is given about the isolation of a non-toxigenic strain of C.diphtheriae belonging to the corresponding cultural and biochemical variant;
- in the presence of precipitation lines on the medium for determining toxigenicity, identical to the lines of the control strain of diphtheria, positive samples for cystinase, urease, glucose and starch fermentation, absence of sucrose fermentation, absence of reduction of nitrates into nitrites, the culture is classified as C.ulcerans, toxigenic variant;
- When diphtheroids are isolated, the answer is negative.
When sown, the pathogen C.diphtheriae is differentiated from other corynebacteria, primarily C.pseudodiphtheriticum, C.ulcerans, C.pseudotuberculosis. To detect diphtheria toxin, RNGA or ELISA methods are used. This study is not mandatory in practical bacteriological laboratories, but can be used as an additional test to provide a preliminary answer. The method also allows you to determine the relative (conditional) quantitative content of the toxin in the test sample.
To identify specific antibodies, the RPGA and RNGA methods are used. The RPGA method is used to study the intensity of anti-diphtheria immunity. The conditionally protective titer of AT is taken to be 1:20.
In patients, the determination of the antibody titer to the antigen of diphtheria bacilli in the blood serum using the RNGA method is carried out in two blood samples collected at the onset of the disease and after 7–10 days. An increase in AT titer by 3–4 times in the second serum relative to the first indicates a previous infection. The study is used primarily for retrospective diagnosis of diphtheria.
Detection of the toxigenic gene (tox) by PCR is the fastest and most reliable method of laboratory diagnosis, but it does not provide information about the ability of a microorganism to express diphtheria toxin. Cases of non-toxigenic diphtheria strains carrying
Prognosis and prevention
The prognosis is most favorable for localized, mild and moderate forms. But, subject to timely treatment using APDS. The most dangerous and less favorable are toxic forms of the disease, late initiation of treatment and the development of complications.

Prevention of diphtheria consists of timely vaccination. The first vaccination is given to children at the age of 3 months, subsequent ones at 9 - 12 months, then 7 years, 12 years and adolescents at 16 years of age. Adults can also get vaccinated. Immunity after vaccination and illness lasts for 10 years. Mass vaccination of the population has reduced the mortality rate from diphtheria to <5%. Previously it reached 60%.
TAKING MATERIAL FOR BACILLUS LOEFFLER (BL)
Indications. Isolation of the pathogen from persons with suspected diphtheria to confirm the diagnosis and examination of contacts; examination of bacteria carriers after their sanitation, as well as patients with sore throats in the presence of plaque, stenotic laryngotracheitis, mononucleosis, peritonsillar abscess; examination of children newly admitted to orphanages, boarding schools, specialized institutions for children with central nervous system damage and tuberculosis; examination of children subject to surgical intervention for ENT pathology.
General information. A swab from the throat should be taken before taking antibiotics, other medications, rinsing the oropharynx with antiseptic solutions, and before brushing your teeth. The material is taken on an empty stomach or no earlier than 2 hours after a meal with immediate inoculation on a Petri dish with a nutrient medium. If this is not possible, immediately send the collected material to the laboratory. The seeding rate increases when taking material at the interface between the films and healthy tissue. When transporting over long distances, enrichment media or tampons moistened with a 5% solution of glycerin in an isotonic sodium chloride solution are used. Delivery to the laboratory should be made no later than 3 hours after taking the material.
Material support: 1) hermetically sealed sterile test tubes (2 pcs.) with cotton swabs on a wooden rod in a box or craft bags; 2) spatula in a craft bag;

Preparatory stage of performing the manipulation.
1. Wash and dry your hands, put on gloves.
2. Place the necessary equipment on the tool table.
3. Using a glass grapher, mark the number on the test tubes corresponding to the number in the direction. Place the test tubes in a rack.
4. Sit the child near the light source and invite him to open his mouth wide. Young children are secured by an assistant.
The main stage of the manipulation.
5. With your left hand, press the spatula onto the root of the tongue.
6. Use a tampon to remove mucus from the tonsils and arches at the border of the affected area and healthy mucous membrane, make sure that the tampon does not come into contact with the mucous membrane of the mouth and teeth.
7. Place the swab into the test tube without touching its outer wall.
8. Take a second tampon and insert it deep into the nasal passage.
9. Make several rotational movements.
10. Carefully remove the swab without touching the skin of the nose and place it in the second tube.
The final stage of the manipulation.
11.Wash and treat gloved hands with an antiseptic solution, remove gloves. Wash and dry your hands.
12. Complete a referral.
13.Transport the collected material in a container to the bacteriological laboratory.
Advantages of JSC "SZDCM"
You can get tested for diphtheria and other bacterial infections in one of the departments of the Northwestern Center for Evidence-Based Medicine. All conditions are created for you here:
- Friendly staff and no queues.
- Qualified laboratory technicians and precise, modern equipment.
- Quick availability of results and several ways to obtain them.
Laboratory terminals and medical centers are conveniently located for travel by public transport and private cars. You can take the study in St. Petersburg and other cities of the Leningrad Region, Veliky Novgorod and the Novgorod region, Kaliningrad and Pskov.