Authors: Robert Lee, Carolyn Dupuis, Byron Chen, Andrew Smith, Young H. Kim
Epidemiology
In pregnant women who were admitted to the ED with abdominal pain and/or vaginal bleeding, the incidence of VD ranged from 6 to 16%.
The main risk factor for ectopic pregnancy is pelvic inflammatory disease, and other high-risk factors include previous ectopic pregnancy and tubal surgery. Additional moderate to low risks are observed with intrauterine device use, multiple sexual partners, smoking, and assisted reproductive technologies, including in vitro fertilization (IVF).
Approximately 98% of ectopic pregnancies occur in the fallopian tubes. Among them, 70% of tubal ectopic pregnancies occur within the ampullary portion, followed by the isthmus, fimbriae, and interstitial tubal segments. Remaining ectopic pregnancies can be found in various locations outside the fallopian tubes, including the ovaries, C-section scars, cervix, and abdomen.
Recovery and rehabilitation
Before you start planning a pregnancy, you must undergo a course of rehabilitation measures aimed at restoring reproductive function. It is important to normalize hormonal levels and prevent the formation of adhesions.
After an ectopic pregnancy, women are recommended to undergo a course of physiotherapeutic procedures. The doctor also prescribes anti-inflammatory drugs and drugs aimed at strengthening the immune system.
Combined oral contraceptives may be prescribed for 3-6 months.
Visualization technique
Ultrasound is the imaging modality of choice for women with symptoms of VD. Ideally, pelvic ultrasound for these patients includes both transabdominal and transvaginal assessment. Transabdominal assessment (Fig. 1A, B) is performed using a low mid-frequency transducer (1-5 MHz) after adequate bladder filling. It includes a wider field of view of the pelvis and provides better visualization of the uterine fundus and superiorly positioned adnexa. It can also be used to visualize free fluid or hemorrhage in the abdomen.
Figure 1: A, B. Transabdominal sagittal and transverse views of the pelvis showing a normal uterus and bladder.
Transvaginal examination (Fig. 2A, B) is performed using a high-frequency intravaginal probe (> 7 MHz) after urination. It provides excellent near-field resolution, allowing detailed assessment of the endometrial cavity. Transvaginal ultrasound also provides a more detailed assessment of the ovaries and other adnexal structures.
Figure 2: A, B. Transvaginal sagittal and transverse views allow better visualization of the endometrium. The intrauterine gestational sac is visible with the yolk sac.
Abnormal early MB
The timing of marker imaging in early pregnancy—PU at approximately 5 weeks' gestation, yolk sac at 5.5 weeks, and embryo at 6 weeks with ±0.5 week variation—is accurate and consistent. Thus, any deviation from this expected time may indicate a pregnancy that is not progressing. Time-based discriminatory values for absence of cardiac activity at a certain CTE, absence of an embryo at a certain SDP, and nonvisualization of a living embryo were established in the 1980s when endovaginal ultrasound was first performed. The criteria were based on small cohorts and originated in single-center academic centers at a time when interoperator variability and standard deviation in measurements were not widely used. More recently, reports of large population-based studies performed on heterogeneous imaging groups have shown greater variability. In addition, modern treatment of ectopic pregnancy has shifted to the use of non-surgical therapy. The use of methotrexate instead of surgery does not confirm the diagnosis made by ultrasound and can also potentially damage the MB. With the goal of absolute certainty of pregnancy loss before irrevocable medical or surgical treatment, the Society of Radiology in Ultrasound consensus group in 2012 revised the traditional discriminatory values to establish more conservative criteria for definitive pregnancy loss and suspected miscarriage.
Ultrasound signs of miscarriage:
— CTE >= 7mm without signs of cardiac activity;
— SDPJ >= 25 mm, PJ without signs of an embryo;
— Absence of an embryo with signs of cardiac activity 2 weeks after an ultrasound scan, which revealed a PU without a yolk sac;
— Absence of an embryo with signs of cardiac activity 11 days or more after ultrasound, which revealed a uterine yolk sac.
Ultrasound signs suspicious for, but not definitively, miscarriage:
— CTE < 7mm without signs of cardiac activity;
— SDPJ 16-24 mm, PJ without signs of an embryo;
— Absence of an embryo with signs of cardiac activity on days 7-13 after ultrasound, which revealed a PO without a yolk sac;
— Absence of an embryo with signs of cardiac activity on days 7-10 after ultrasound, which revealed a polyp with a yolk sac;
— Absence of an embryo at 6 weeks or more of gestational age (counting from the first day of the last menstruation);
— Empty amnion (next to the yolk sac, without an embryo), enlarged yolk sac (> 7 mm), small size of the PU in relation to the size of the embryo (difference < 5 mm between SDPJ and CTE).
For many years, an empty uterus (without a yolk sac) measuring 8 mm or more was considered diagnostic of miscarriage, but this criterion is now considered too narrow and has been abandoned.
Previously, a CTE of 5 mm without cardiac activity met the criterion for miscarriage; however, in one series this resulted in a false-positive rate of 8.3%. There have also been reports of embryos with a CTE of 6 mm and no cardiac activity that subsequently resulted in a viable pregnancy.
Because of interoperator variability in CTE measurements on TVUS, a 7-mm CTE is required to provide a specificity and positive predictive value of 100%, thereby reducing the likelihood of false-positive diagnoses associated with the 5-mm CTE threshold.
The same applies to the use of the 25 mm TDP cut-off without an embryo as a criterion for miscarriage, and to the previously used 16 mm TDP and 16–24 mm TDP range without an embryo as an indicator of suspected miscarriage.
Using a 16-mm PDU as the threshold for diagnosing miscarriage resulted in a false-positive rate of 4.4% in one series of studies where PPUs with a mean diameter of 17 to 21 mm and no visible embryo subsequently resulted in viable pregnancies. Due to inter-operator variability in TVUS measurements, a 25 mm cut-off for DPJ increases specificity to 100%. Not all failed or potentially non-viable intrauterine pregnancies demonstrate a 7-mm CTP without cardiac activity or a 25-mm SDP without an embryo, requiring additional criteria based on non-visualization of a living embryo after a certain period of time.
Morphological assessment of individual components of pregnancy, including the uterus, yolk sac, amnion, embryo, cardiac function and decidua, helps assess pregnancy prognosis. Additional criteria that are suspicious for miscarriage in the consensus criteria include an empty amniotic sac, an enlarged yolk sac, and a small gestational sac size relative to the size of the embryo. Given that the length of the amniotic cavity is equal to the CTE during 6.5–10 weeks of gestation in a normal pregnancy, the presence of an “empty amnion” with no identifiable embryo adjacent to the yolk sac is a sign of poor prognosis and requires follow-up ultrasound. An enlarged yolk sac measuring more than 7 mm and small PU size relative to embryo size (defined as a difference between DPTU and CTE of less than 5 mm) are also associated with poor pregnancy outcome. A PO with an irregular contour (lack of a smooth contour and/or the presence of a distorted shape of the PO) is also a criterion suspicious for an abnormal pregnancy. In one series, this result had 100% specificity and 100% positive predictive value for abnormal MB, but it had a low sensitivity of 10%. The presence of a calcified yolk sac suggests that embryonic death is likely to be of relatively long duration, 2 weeks or more. An enlarged or dilated amnion (amnion too large for the size of the embryo), fetal bradycardia of 85 beats per minute or less, degenerative hydropic changes in the chorionic villi (*which are sometimes mistaken for retrochorial hematomas), and an amorphous shape of the fetus at 7–8 weeks of gestation are also signs poor prognosis and requires follow-up ultrasound.
Retrochorial hemorrhage occurs in 18–22% of first-trimester pregnancies with vaginal bleeding. The clinical significance depends on the size of the hematoma. The risk of pregnancy loss doubles with large hematomas, especially when there is a circumference greater than two-thirds of the circumference of the chorion. Chorionic tubercle, thought to represent a small hematoma on the choriodecidual surface that protrudes into the gestational sac, is a controversial finding and is associated with a guarded prognosis, but a more recent study has demonstrated a more questionable prognosis (*see - https://www.uzgraph.ru/ daydzhest/4/824/05-09-2019-… ).
Clinical and diagnostic approach
Any premenopausal woman experiencing abdominal pain and/or vaginal bleeding should take a pregnancy test. Serum β-human chorionic gonadotropin (β-hCG) is a more sensitive test than urine hCG for confirming pregnancy, and a negative serum β-hCG virtually rules out pregnancy. Patients with a positive pregnancy test and symptoms suggestive of an ectopic pregnancy are then examined with a pelvic ultrasound to determine the presence or absence of an ectopic pregnancy.
If intrauterine pregnancy is not detected by ultrasound, ultrasound findings should be carefully interpreted in the context of the patient's clinical information, particularly the expected gestational age according to the last menstrual phase and the serum β-hCG level relative to the discriminatory zone. The discriminatory zone is the serum β-hCG level, above which intrauterine pregnancy (IP) is expected to be visualized by ultrasound. For transvaginal ultrasound, the discriminatory zone in most institutions ranges from 1500 to 2000 mIU/ml. The discriminatory zone is higher for transabdominal ultrasound, 6000-6500 mIU/ml.
If there is no sonographic confirmation of MB in patients with β-hCG levels above the discrimination zone, an alternative source of β-hCG should be considered. Bilateral adnexa should be examined carefully, as ectopic pregnancies can have variable, and often subtle, sonographic findings. Even in such patients with apparently normal adnexa, ectopic pregnancy should be included in the differential diagnosis.
DO YOU CARE CORRECTLY FOR YOUR ULTRASOUND DEVICE?
Download your care guide now
Download PDF
About the ultrasound procedure
Ultrasound examination is an imaging technique based on the use of high-frequency sound waves, which make it possible to obtain cross-sectional images of internal organs and various parts of the body. The diagnostic ultrasound apparatus consists of the following components:
- monitor;
- keyboard;
- CPU;
- data storage device;
- sensor, also known as converter.
The transducer emits ultrasound waves at a given frequency and picks up reflected sounds at frequencies that depend on the tissue through which the waves pass. The ultrasonic wave returning to the transducer is digitized, and an image appears on the monitor in the form of dots or echo signals. All images are examined in real time and can be obtained in any image plane.
Ultrasound diagnosis of ectopic pregnancy helps to determine pathology from the 5th week after conception. Already in such a short period of time, the procedure will show the location of the fertilized egg and help determine its location if it is located outside the uterus. Timely diagnosis and treatment of pathology help prevent serious complications and preserve women's health and reproductive function.
Ultrasound results for tubal ectopic pregnancy
Tubal ectopic pregnancy most often appears as an extraovarian heterogeneous mass, usually presenting as a hematoma at the site of ectopic implantation (Figure 3). The mass may exhibit varying degrees of echogenicity depending on the age of the blood products.
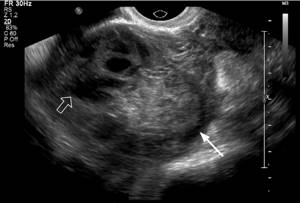
Figure 3: An ectopic pregnancy is considered a hematoma. This 21-year-old woman with a positive serum pregnancy test and vaginal bleeding in the right adnexa shows a complex echogenic mass (arrow) that is separated from the right ovary (open arrow) with pressure applied during transvaginal ultrasound. An echogenic adnexal mass is typical of a hematoma at the site of ectopic implantation. The patient was treated surgically.
A tubal ectopic pregnancy may also be seen as an echogenic adnexal ring surrounding an unruptured ectopic pregnancy, which is known as a tubal ring sign (Figure 4A). It is the second most common sign of ectopic pregnancy and has a 95% positive predictive value. Color Doppler often reveals a ring of peripheral vasculature (Figure 4B).
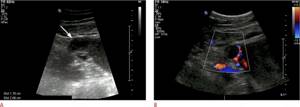
Figure 4: Pipe-ring sign. This 20-year-old woman with a positive pregnancy test presenting to the emergency department with pelvic pain and vaginal discharge has an adnexal mass with an echogenic ring (arrow). B. Color Doppler image of the right adnexa shows increased vascularity in the echogenic ring. The patient was diagnosed with an ectopic pregnancy based on clinical and sonographic evaluation and was successfully treated with methotrexate.
Visualization of the extrauterine gestational sac with the embryo (Fig. 5A, B) directly confirms an ectopic pregnancy, but this fact is rarely observed.
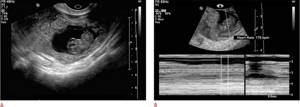
Figure 5: Live tubal ectopic pregnancy. A. This 25-year-old woman with a positive pregnancy test and vaginal bleeding had a gestational sac with an embryo in the left adnexa, outside the left ovary. B. M-mode ultrasound of the fetal left adnexa shows a fetal heart rate of 170 beats per minute. These findings confirm a living tubal ectopic pregnancy.
Additional sonographic findings are associated with ectopic pregnancy. The presence of free intraperitoneal fluid (Figure 6) in patients with a positive pregnancy test and an empty uterus has 69% specificity and 63% sensitivity for ectopic pregnancy. While free fluid is a nonspecific finding that can be observed both physiologically and in other pathologies, a moderate to large volume of free fluid is greater than expected for physiological free fluid volume and complexity, with floating debris, blood products, and/or organized thrombi ( Fig. 7) are suspicious signs of ectopic pregnancy.
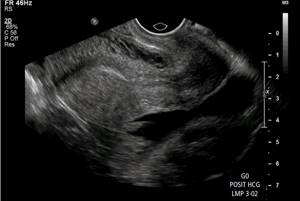
Figure 6: Simple free liquid. A 21-year-old woman presenting to the emergency department with pelvic pain and a positive pregnancy test was found to have simple free fluid in her pelvic cavity. The patient also had an echogenic left adnexal mass (not included in this figure) that was confirmed as an ectopic pregnancy during surgery.
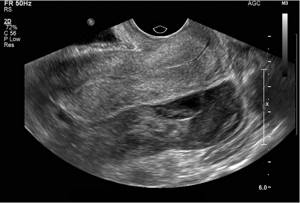
Figure 7: Free fluid complex or hemoperitoneum. This 22-year-old woman with a previous history of ectopic pregnancy presented to the emergency department with pelvic pain and a positive pregnancy test was found to have a large volume of complex free fluid with internal echogenicity in the pelvic cavity, most likely representing hemoperitoneum. An ectopic pregnancy with tubal rupture was confirmed during surgery.
The so-called pseudogestational sac is a small amount of intrauterine fluid (Figure 8A) that can be misinterpreted as a true gestational sac in cases of ectopic pregnancy (Figure 8B). It is estimated to occur in 10% of patients with ectopic pregnancy. Real-time observation of this phenomenon often shows fluid displacement during the examination, as opposed to the fixed position of the true intrauterine gestational sac.
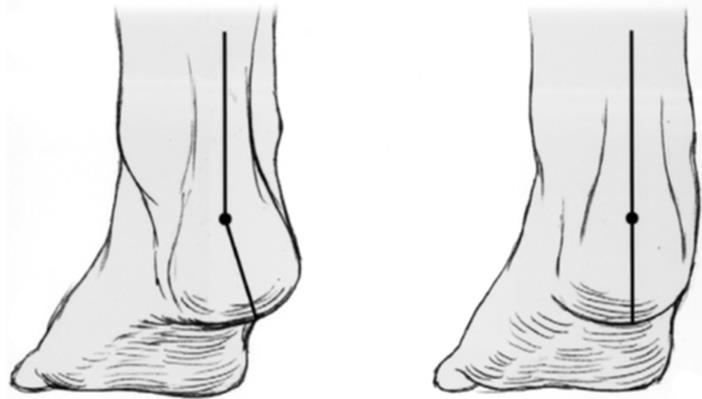
Figure 8: Pseudogestational sac in ectopic pregnancy. A 28-year-old woman with a positive pregnancy test presented to the emergency department with right lower quadrant pain. A. A small amount of free fluid is visible in the endometrial cavity, with no evidence of a yolk sac or embryo (arrow). It is irregularly shaped and centrally located rather than in the eccentric location often seen with a normal gestational sac. However, it should be noted that in a woman who tests positive for β-human chorionic gonadotropin (β-hCG), any intrauterine sac-like fluid seen on ultrasound is likely to be a gestational sac. B. There is a right adnexal mass with an echogenic ring (open arrow), consistent with an ectopic pregnancy. This patient was clinically evaluated with serial β-hCG testing and was later diagnosed with ectopic pregnancy and treated medically.
Diagnostics
First, the obstetrician-gynecologist collects anamnesis and asks the woman the necessary clarifying questions. As part of the examination, the doctor identifies a space-occupying formation to the left or right of the uterus. The doctor also correlates the gestational age with the size of the uterus.
In addition, the following studies are prescribed:
- Ultrasound of the pelvis, during which the embryo in the uterine cavity is not visualized;
- blood test for hCG (an increase in the concentration of the hormone according to the results of several tests in the absence of a fertilized egg in the uterine cavity indicates an ectopic pregnancy);
- diagnostic laparoscopy (if necessary).
Other rare types of ectopic pregnancies
In interstitial ectopic pregnancy, implantation occurs in the interstitial part of the fallopian tube, which accounts for 2%-4% of ectopic pregnancies. Interstitial pregnancy can be particularly dangerous due to the high risk of profuse hemorrhage due to its propensity for delayed, larger rupture and its proximity to the intramyometrial arcuate vasculature. On ultrasound, it may be difficult to distinguish a conventional MB, which is eccentrically located. Diagnosis is suggested when the gestational sac is visualized high at the fundus, surrounded by a thin layer of myometrium measuring less than 5 mm. The interstitial line sign is an echogenic line extending from the endometrium to the ectopic gestational sac.
Ectopic pregnancies with cesarean section account for less than 1% of ectopic pregnancies. The gestational sac is found in the myometrium of the anterior lower segment of the uterus at the site of a previous cesarean section scar. Often there is severe thinning of the myometrium or complete absence of myometrium between the gestational sac and the bladder. A scarred ectopic pregnancy significantly increases the risk of uterine rupture.
Cervical ectopic pregnancy occurs in less than 1% of ectopic pregnancies. Diagnosis is made when the gestational sac is visualized in the cervical stroma, usually in an eccentric position. The resulting stretching of the cervix results in an hourglass-shaped uterus.
Ovarian ectopic pregnancy refers to intracavitary implantation of the gestational sac and can account for up to 3% of ectopic pregnancies. If an adnexal mass is detected, a gentle bimanual ultrasound examination should be performed to ascertain the location of the mass in relation to the ovary, as the intracavitary mass will move with the ovary with applied pressure. However, an anechoic or even complex ovarian mass is more likely to be a corpus luteum cyst, since ovarian ectopic pregnancies are rare.
Abdominal/peritoneal pregnancy is an ectopic pregnancy in the abdominal cavity, accounting for approximately 1% of ectopic pregnancies. It is most often found in the space of Douglas, but can be located anywhere in the abdomen. This is a complex diagnosis that is not easy to make using imaging and is often made intraoperatively.
Heterotopic pregnancy is a rare disease with two simultaneous pregnancies: normal MB and VB. Heterotopic pregnancy has a significantly higher incidence among patients receiving IVF, compared to the trivial incidence among patients who conceive naturally of 1:30,000. Timely diagnosis and treatment can be critical not only in reducing maternal morbidity and mortality, but also to save MB.
What happens during ectopic embryo development
According to clinical manifestations, ectopic pregnancy is of two types:
- Progressive
. In the early stages, spontaneous miscarriage does not occur, so the fertilized egg continues to grow, then growing into the wall of the fallopian tube. The manifestations of a progressive ectopic pregnancy are similar to normal ones. Until the moment of ingrowth, a woman sometimes does not feel any changes in her condition; sometimes there is bloody discharge from the vagina, similar to menstrual discharge. This is often confused with scanty menstruation. - Interrupted
. It ends with spontaneous miscarriage, but is divided into subtypes based on the type of development.
Tubal abortion
. The embryo detaches from the wall of the uterus and exits into the abdominal cavity. The patient has blood clots from the vagina, there is sharp pain in the lower abdomen, it hurts to sit and even lie down. The uterus is enlarged on palpation, the appendages are dilated.
Pipe rupture.
Occurs between 6-10 weeks of pregnancy. An embryo that grows into the fallopian tube ruptures the organ, causing severe bleeding. The woman experiences a stabbing pain in the lower abdomen, radiating to the lower back. The patient lies only on her left side with her legs tucked to her stomach. Her posterior vaginal vault bulges and her cervix moves forward. The patient loses consciousness due to a decrease in blood pressure, cold sweat appears.
Diagnosis of an interrupted ectopic pregnancy allows you to avoid serious health consequences and preserve the female reproductive system.
Ultrasound results after treatment
Methotrexate has been shown to be a safe and effective treatment for ectopic pregnancy. After treatment with methotrexate, ultrasound is indicated only if tubal rupture is suspected based on persistent clinical symptoms or hemodynamic instability. Sonographic findings after medical treatment can be misleading, as there is often a paradoxical increase in ectopic pregnancy due to bleeding and swelling after treatment (Fig. 10A, B). An ectopic mass/hematoma may be visible more than 2 months after treatment. In the context of clinical improvement and a corresponding decrease in β-hCG levels after medical treatment, these sonographic findings usually represent the expected resolution of the ectopic pregnancy.
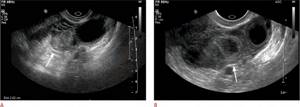
Figure 9: Sonographic findings after treatment with methotrexate. This is a 31-year-old woman with a positive but declining beta human chorionic gonadotropin level. A. There is a heterogeneous echogenic mass in the left adnexa (arrow) adjacent to the left ovary (open arrow). The patient was diagnosed with a left tube ectopic pregnancy and was started on methotrexate. B. On a follow-up pelvic ultrasound 10 days after starting methotrexate treatment, there is an increase in the left tube ectopic pregnancy interval (arrow) due to surrounding bleeding and swelling.
Areas of fetal location in pathology
Implantation of a fertilized egg during an abnormal pregnancy most often occurs in the following areas:
- pipe;
- ovarian;
- cervical;
- peritoneal.
During a gynecological examination, the doctor can roughly guess where the fertilized egg is attached. But the final diagnosis will be determined by ultrasound results, so after the initial examination, the doctor gives a referral for this informative and safe type of diagnosis.
Pipe zone
Implantation of a fertilized egg in the cavity of the fallopian tubes is the most common type of ectopic pregnancy. Pathologies of the fallopian tubes in the form of adhesions, scars, and obstruction become the main reason that the fertilized egg cannot reach the uterus and attach to its cavity. Implantation in this situation occurs in the fallopian tube. After attachment, the embryo continues to grow and develop, gradually stretching and injuring the tissues of the organ. The danger lies in the rupture of the pipe and the development of internal uncontrolled bleeding, which, if medical care is not provided on time, can cause death. This is why ultrasound for early ectopic pregnancy is such a valuable diagnostic method.
Abdominal space
Engraftment of a fertilized egg in the peritoneal zone is relatively rare. There it attaches to the wall of any organ, after which it begins to actively grow and develop, disrupting normal functioning. With this pathology, the option of having a child is possible, but the woman will have to face many difficulties. A fetus developing in an uncharacteristic place is unprotected from the negative effects of internal and external factors. Therefore, at any time, it may freeze and miscarriage.
Cervical zone
In this case, the fertilized egg is attached to the wall of the cervix. This form of ectopic pregnancy is relatively rare, but it is very dangerous for a woman’s life. With cervical pathology, there is a risk of damage to large blood vessels located in this area. If this happens, widespread, uncontrolled bleeding occurs, which can be fatal.
Ovarian zone
The embryo in ovarian pathology develops in the ovary itself, which, as it grows and develops, leads to rupture and extensive bleeding. There are 2 subtypes of ovarian pregnancy:
- intrafollicular;
- epioformal.
In the first case, implantation occurs in the follicle, and in the second - on the surface of the ovary.
Ultrasound-like conditions with ectopic pregnancy
The corpus luteum may look very similar to an ectopic pregnancy on ultrasound. It is usually a round, thick-walled structure, often cystic, with variable internal echogenicity depending on the presence of hemorrhage (Fig. 10A). Peripheral vascular flow is noted on the cyst wall and is visible as a “ring of fire” on color Doppler (Figure 10B). The most reliable way to distinguish the corpus luteum from an ectopic pregnancy is to determine the location of the lesion relative to the ovary, since VT occurs in the ovary, and the vast majority of ectopic pregnancies are tubal (extraovarian).
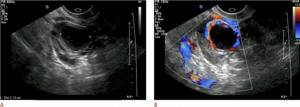
Figure 10: This figure shows a 32-year-old woman who presented to the emergency department with lower abdominal pain. A. There is an intracavitary cystic structure with an echogenic ring and internal debris (indicated by cross marks). This ovarian structure is most likely the corpus luteum. B. Color Doppler ultrasound of the same structure shows significant peripheral vascularization, which is often observed in the corpus luteum.
An occasional adnexal mass may be mistaken for an ectopic pregnancy. Paraovarian cysts (Fig. 11) are usually simple unicellular adnexal cysts outside the ovary that are often found incidentally. A paraovarian cyst located adjacent to the ovary may raise concern for an ectopic pregnancy, but its appearance as a simple cyst with a thin avascular wall easily distinguishes it from an ectopic pregnancy.
Dermoid cysts (Fig. 12A,B) can have a variety of ultrasound appearances but are usually seen as a focal hyperechoic mass in the ovary. They may also have echogenic dew-like shadow nodules, fluid levels, calcifications, or dot-dash-like dots due to hair. Ovarian neoplasms, although less common than paraovarian cysts or dermoid cysts, can also mimic an ectopic pregnancy. If there is concern about an ovarian mass based on the patient's clinical presentation and ultrasound findings, an MRI may be performed to further evaluate the mass.
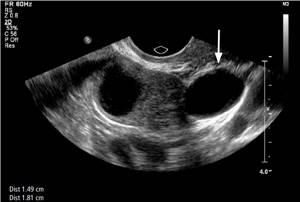
Figure 11: Paraovarian cyst. A 47-year-old woman has an anechoic cyst with a thin wall and posterior acoustic enhancement below the right ovary, consistent with a paraovarian cyst (arrow).

Figure 12: Dermoid cyst. This is a 17-year-old girl who presented to the emergency department with pelvic pain. A. Complex echogenic mass with posterior acoustic shadowing (arrow). B. Several thin echogenic streaks, also known as stippling (open arrow), are visible in parts of the mass representing hair follicles. These findings are compatible with a dermoid cyst
Visualization of bowel loops in the pelvis (Figure 13) can sometimes be misinterpreted as an ectopic pregnancy, and vice versa. Loops of intestine may appear as a mass-like structure with distinct walls and vascularity. Real-time images taken by a radiologist or Cine images provided by a technologist can be very helpful in differentiating the bowel from an ectopic pregnancy by demonstrating peristalsis in the bowel loops.
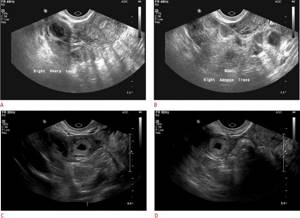
Figure 13: This figure shows a 28-year-old woman who presented to the emergency department with right lower quadrant pain and a positive pregnancy test. A. Sagittal view of the right adnexa shows a hemorrhagic cyst in the right ovary; otherwise, no definite appendage mass is visible. B. Transverse image of the right adnexa did not show a definitive mass in the right adnexa. During the study, nonspecific echogenicity in the right adnexa was interpreted as intestinal loops. C, D. The patient returned 3 days later with intermittent pain in the right lower quadrant. There is a mass with a thick echogenic ring in the right adnexal body, which is highly suspicious for an ectopic pregnancy. The diagnosis of tubal ectopic pregnancy was confirmed during surgery.
Why does ectopic pregnancy happen: there are too many reasons
Let's look at why ectopic pregnancy occurs.
Most often, anatomical reasons prevent a fertilized egg from reaching its destination:
- Pathological changes in the fallopian tubes
(55% of cases). Caused by scars and adhesions (salpingitis) - a consequence of inflammatory processes, for example, adnexitis, hydrosalpinx. Scars prevent the walls of the appendages from contracting, and adhesions block the tubal lumen. The pipe itself is deformed, changing its contours and dimensions. Scars and adhesions also appear after STIs, abortions, hypothermia, etc. They are the most common causes of ectopic pregnancy in the early stages. - Intrauterine devices
(intrauterine devices) are the cause in 4% of cases. If installed incorrectly and worn for too long, atrophy of the ciliated epithelium is provoked and the mucous membrane is damaged.
External factors include:
- Consequences of surgery
. Laparoscopic surgery to remove uterine fibroids, ovarian cysts, or cesarean section often leads to scar formation or inflammation, which causes pregnancy outside the uterus. - Reconstruction of fallopian tubes in case of obstruction.
- Tumors on the uterus or ovaries, leading to deformation of the tubes.
- Genital infantilism
. This is a discrepancy between the development of the genital organs and the biological age of the woman. The pathology occurs at the stage of embryonic development of the child, and manifests itself in the underdevelopment of the reproductive organs. Girls with sexual infantilism have a childish figure with undeveloped hips and breasts. They have shortened fallopian tubes, and uterine hypoplasia is often diagnosed.
Is the man to blame for an ectopic pregnancy? There is no direct connection between sperm quality and pregnancy outside the uterus. But there is an indirect factor here - a genitourinary infection, which led to inflammation of the appendages.
Hormonal causes that accumulate with age:
- Disturbances in the functioning of the hypothalamus and adrenal glands;
- The use of hormonal drugs in excessive doses in the treatment of infertility;
- Stimulation of ovulation with hormonal drugs before IVF;
- Impaired production of prostaglandins affects the contractility of the appendages, as a result of which the zygote does not move towards the uterus;
- Increased enzymatic activity of the egg leads to the attachment of the fertilized egg not to the uterus, but to the walls of the fallopian tube.
Psychological causes of ectopic pregnancy
If an ectopic pregnancy is detected in a therapeutically healthy woman without obvious reasons, then doctors talk about psychosomatic factors. The stress hormones norepinephrine and cortisol disrupt the production of progesterone and estrogen, which control the menstrual cycle. As a result of hormonal imbalance, the mucous surface of the lumen of the fallopian tubes is damaged, and the function of transporting the fertilized egg to the uterus is disrupted.
Psychosomatics studies the patterns of influence of a person’s psychological state on health. Suppression of negative emotions, decisions made against the will, experiences that have not found a way out take on physical form. In women, the reproductive sphere is vulnerable, which is the first to suffer.