K is the main cation of intracellular fluid, which contains 98% of the potassium of the whole body. The cation ensures osmolarity of the cytoplasm and creates conditions for biochemical reactions to occur in it. The entry of K into the cell through the plasma membrane is determined by many factors. Potassium channels provide passive permeability of the membrane for the cation, while the movement of K is determined by the magnitude of the electrical potential and the concentration gradient. Potassium channels can be in an open or closed state; the passive flow of potassium into the cell is determined precisely by the number of currently open channels. The high permeability of cell membranes for K, especially in a state of excitation, and “relative impermeability” for Na, leads to the emergence of a potential difference on the plasma membrane.
The stability of K content in the body is a consequence of balancing the processes of its intake and excretion. The intake of K from food is most important in the regulation of the total cation pool. An adult consumes 80–100 mmol K daily. From 85 to 90% of K taken in food is excreted by the kidneys. In the absence of K in food, 40–60 mmol/day is excreted in the urine for several days, after which the loss of K in the urine decreases to 10–20 mmol/day. 10–15% of K consumed with food is excreted through the gastrointestinal tract. This pathway of cation excretion may be enhanced in conditions of mineralocorticoid hypersecretion and renal failure. A significant amount of K can be released through the skin only in case of intense sweating.
Cells of the nervous and muscular systems quickly respond to a decrease in plasma K levels, which explains the early appearance of symptoms - muscle hypotension, weakness and asthenia, weakened reflexes. Some cases of hypokalemia lead to symptoms of paralysis and even rhabdomyolysis. Severe hypokalemia is accompanied by disturbances in conduction and rhythm of cardiac activity, which is reflected in the ECG (decreased wave amplitude, T wave inversion, widening of the S-T interval and the appearance of a U wave). With chronic K deficiency, the size of the heart increases, rhythm disturbances occur, and with a sharp decrease in the level of K in the plasma, cardiac arrest in systole is possible.
Symptoms of hyperkalemia depend on the rate of increase in the cation content in the plasma and are associated with changes in the membrane potential of neurons and muscle cells. Neuromuscular symptoms of hyperkalemia are expressed in paresthesia, and in more severe cases - in ascending paralysis of the limbs. The severity of cardiac symptoms depends on the degree of increase in plasma K - from minimal ECG changes to serious rhythm and conduction disturbances (transverse block and ventricular fibrillation). When the K concentration is above 6 mmol/l, as a rule, ECG changes are detected (sharpening of the T wave, lengthening of the P wave and P-R interval, widening of the QRS complex, elevation or depression of the ST segment), which become more and more pronounced as hyperkalemia progresses. At a K level above 7 mmol/l, the development of cardiac arrhythmia with a fatal outcome was noted; at a K concentration above 10 mmol/l, the heart may stop in diastole. In the clinic, prolonged hyperkalemia is most often a consequence of chronic renal failure.
Potassium in the blood
In clinical biochemistry, K metabolism is assessed based on its content in the blood plasma (intravascular pool), although it contains no more than 2% of the total amount of the cation. The overwhelming majority of K in the body is located inside cells, and its concentration in plasma, which only approximately reflects the total content of the element in the body, can fluctuate within significant limits, especially in severe pathologies. It is closely related to the state of blood circulation and acid-base balance, therefore the determination of K in plasma or serum is one of the most important indicators during the period of resuscitation.
Disturbances in blood pH have a significant effect on the level of K in the blood plasma. Metabolic acidosis causes the movement of hydrogen ions into the cell in exchange for intracellular K, which leads to a decrease in potassium content in tissues. With alkalosis, on the contrary, an increase in the K content in tissues is observed. The level of K in plasma is affected by the content of the HCO3 - anion in the blood, which reflects the activity of catabolic processes.
A decrease in the concentration of potassium in the blood occurs when there is insufficient intake of potassium from food, increased loss by the kidneys (taking diuretics, the diuretic stage of acute renal failure, tubular acidosis, increased secretion of ADH, excess mineralocorticoids, etc.); increased losses due to vomiting or diarrhea, intravenous administration of large volumes of fluid that does not contain K, redistribution of K between extra- and intracellular fluid (acute and chronic metabolic acidosis, acute respiratory alkalosis, administration of insulin or glucose). The most common cause of hypokalemia is increased loss of K by the kidneys; insufficient K intake rarely causes hypokalemia.
Hypokalemia can occur in alcoholics even with a slight increase in Na levels in the blood. Drinking large quantities of beer also leads to hypokalemia and hyponatremia; Beer contains very few salts.
Hyperkalemia can develop in cases of increased K intake (reception of excess K during diuretic therapy, parenteral administration, blood transfusion with expired shelf life), redistribution of K between water spaces (tissue damage, activation of catabolic processes, systemic acidosis), decreased K+ excretion (acute and chronic renal failure, long-term use of potassium-sparing diuretics, mineralcorticoid deficiency).
Hyperkalemia in adrenal insufficiency is accompanied by low levels of Na in the blood plasma, hypokaliuria, and high renin activity. Hyperkalemia with selective aldosterone deficiency is accompanied by low renin levels and impaired cortisol levels in the blood. Disorders of carbohydrate metabolism in acute and chronic renal failure provoke hyperkalemia and metabolic alkalosis.
A common cause of elevated K levels in blood plasma is non-compliance with blood collection conditions. The most common mistake is hemolysis. With a large number of platelets or leukocytes, as occurs in cases of myeloproliferative diseases, pseudohyperkalemia may occur due to increased release of potassium from the cells.
Indications for the study
- Diseases of the cardiovascular system (arrhythmias, arterial hypertension);
- kidney pathology;
- adrenal insufficiency;
- control of therapy with diuretics, cardiac glycosides;
- carrying out hemodialysis.
Features of sample collection and storage:
blood serum without signs of hemolysis. Blood is taken with minimal compression of the vein without muscle load. Samples are stable for no more than 3 hours at room temperature.
Research method:
Determination of the concentration of potassium ions in the blood is currently carried out mainly by the ion-selective method.
Reference interval:
3.5 – 5.1 mmol/l
Increased values
- Hypoaldesteronism primary and secondary;
- acute renal failure;
- taking ACE inhibitors; potassium-sparing diuretics;
- diabetic ketoacidosis;
- increased breakdown of cells and tissues (intravascular hemolysis, rhabdomyolysis, prolonged fasting, convulsions, severe injuries, deep burns, destruction of tumor cells);
- dehydration.
Reduced values
- Primary and secondary hyperaldosteronism;
- hypercortisolism;
- renal dysfunction;
- increased loss of potassium through the gastrointestinal tract;
- hyperglycemia;
- hypothermia;
- lack of potassium in the body (chronic starvation, anorexia).
Basic electrolytes
Potassium
- an important component of most cells. Together with other electrolytes, potassium ions are responsible for the functioning of muscles and nerves, normal acid-base balance, and water metabolism. The blood contains only a small amount of the macronutrient; even minor fluctuations in its level lead to serious consequences. Significant deviations from the norm can lead to life-threatening conditions (shock, cardiac dysfunction, respiratory failure, etc.). Normal potassium concentration is 3.5-5.1 mmol/liter.
Magnesium
participates in energy production, enzyme synthesis, muscle contraction and other vital processes. It is absorbed from food into the gastrointestinal tract and excreted by the kidneys. A deficiency of this electrolyte can be caused by poor nutrition, problems with its absorption, or excessive loss in urine. Elevated levels may indicate kidney pathologies. Significant electrolyte deficiency leads to symptoms such as weakness, confusion, loss of appetite, and muscle spasms. Its excess manifests itself in a similar way. Heart rhythm disturbances and nausea may also be added to the symptoms.
Sodium
present in all tissues and fluids of the body. It is necessary for muscle contraction and maintaining water-salt balance. The macroelement is absorbed in the intestines from ordinary table salt. Deviations of its normal level are associated with a violation of one of the mechanisms of its maintenance. For example, normal sodium concentrations are disrupted if antidiuretic hormone, which prevents fluid loss through urine, is produced in abnormal amounts. A violation of the amount of this electrolyte leads to the appearance of edema or dehydration, as the amount of fluid in the tissues changes. Sodium testing is used in the diagnosis of many diseases (for example, pathologies of the kidneys, lungs, brain).
Chlorine
It is part of many biologically active substances and performs a number of physiological functions. Its level is normally relatively stable (a slight decrease in levels is observed after eating). A test for the amount of chlorine is often prescribed in conjunction with other studies to identify various pathologies. Its results are also used to determine the cause of weakness, prolonged vomiting, diarrhea, and breathing problems.
2. Reasons
It would be logical to assume that hyperkalemia is caused by excessive consumption of potassium-rich foods, for example, dried fruits, legumes, potatoes, bananas, etc. However, in reality this is extremely rare: the body’s daily need for potassium is quite large (up to 5 g in adults), and with normal metabolism the balance is easily restored through the excretion of potassium in the urine.
Thus, one of the main factors in the development of hypercalcemia is disorders of the kidneys (chronic renal failure, diabetic nephropathy, etc.), when potassium excretion becomes difficult and it begins to be retained in the body. This can be caused, in particular, by hypocorticism (Addison's disease) - chronic functional insufficiency of the adrenal cortex, in which the kidneys simply do not receive the appropriate hormonal “commands”.
Another group of reasons is associated with long-term use of medications that contain potassium or prevent its excretion.
The massive release of potassium from cellular fluids into the bloodstream is accompanied by severe polytrauma with crushing of large volumes of muscle tissue, drug overdose, and extensive burns.
The progressive accumulation of potassium in the body can also be caused by pathologically rapid and intense hemolysis (decomposition of red blood cells) in anemia and some other diseases.
Rarely, hereditary hyperkalemia is recorded - familial hyperkalemic periodic paralysis.
Visit our Therapy page
3. Symptoms and diagnosis
The clinical picture of hyperkalemia is not specific enough, so preliminary, presumptive diagnosis requires a doctor to have a lot of experience, attention and vigilance in this regard. Most often, changes in the ECG in combination with patient complaints of tachycardia (accelerated heartbeat), general malaise and constant weakness cause alarm. In more severe cases, cardiac abnormalities caused by excess potassium may take the form of arrhythmia, asystole, ventricular fibrillation, and ultimately cardiac arrest.
The diagnosis is established by comparing the existing clinical manifestations, the electrocardiographic picture and the results of laboratory tests (determining the concentration of potassium in the blood plasma). Depending on the most likely causes, an additional specialized examination is prescribed - for example, nephrological or endocrinological.
About our clinic Chistye Prudy metro station Medintercom page!
4.Treatment
With moderate and severe severity, hyperkalemia requires emergency intensive care due to the threat of cardiac arrest. One of the emergency measures is the intravenous administration of a special solution (calcium, insulin, glucose), but its effect is short-lived, and after relief of life-threatening symptoms, further therapy is required. Potassium-absorbing sorbents are prescribed (which ensures its excretion in feces); diuretics are used if renal function is preserved. Stop taking any medications that in one way or another affect the circulation of potassium in the body. Limit potassium intake from food. Since hyperkalemia is almost always secondary, a diagnostic examination (if the cause of the detected hyperkalemia is unknown) and treatment of the underlying disease are mandatory.
Hyperaldosteronism - symptoms and treatment
In order not to miss hyperaldosteronism, it is first extremely important to identify the main risk factors that will help to suspect this disease. These include:
- arterial hypertension of the second degree, i.e. a stable increase in systolic (upper) blood pressure more than 160/179 mm Hg. Art., diastolic (lower) - more than 100/109 mm Hg. Art.;
- arterial hypertension, persistent and/or poorly controlled with medications (although this sign does not always indicate pathology);
- a combination of arterial hypertension with low levels of potassium in the blood (regardless of taking diuretics);
- arterial hypertension and incidentally detected (by ultrasound and/or CT) formation of the adrenal gland;
- burdened family history: the development of arterial hypertension and/or acute cardiovascular accidents before the age of 40, as well as relatives who have already been diagnosed with hyperaldosteronism [1][5].
The next stage of diagnosis is laboratory confirmation . To do this, the aldosterone-renin ratio (ARR) is examined. This study is the most reliable, informative and accessible. It should be carried out in the early morning hours: ideally no later than two hours after waking up. Before drawing blood, you need to sit quietly for 5-10 minutes.
IMPORTANT: Some drugs may affect plasma aldosterone concentrations and plasma renin activity, which in turn alters ARS. Therefore, two weeks before taking this test, it is important to stop drugs such as spironolactone, eplerenone, triamterene, thiazide diuretics, drugs from the group of ACE inhibitors, ARBs (angiotensin receptor blockers) and others. The doctor should inform the patient about this and temporarily prescribe a different treatment regimen for hypertension.
If APC is positive, a confirmatory saline test should be performed. It is carried out in a hospital setting, as it has a number of limitations and requires a study of the levels of aldosterone, potassium and cortisol initially and after a 4-hour infusion of two liters of saline. Normally, in response to a large amount of fluid administered, the production of aldosterone is suppressed, but with hyperaldosteronism it is not possible to suppress the hormone in this way.
Low levels of potassium in the blood are observed in only 40% of cases of the syndrome, so it cannot be a reliable diagnostic criterion. But the alkaline reaction of urine (due to increased excretion of potassium by the kidneys) is a fairly characteristic sign of pathology.
If familial forms of hyperaldosteronism are suspected, genetic typing (susceptibility testing) is carried out with consultation of a geneticist [3][6].
The third stage of diagnosis is topical diagnosis . It is aimed at finding the source of the disease. For this, various methods of visualizing internal organs are used.
Ultrasound of the adrenal glands is a low-sensitivity diagnostic method. CT is preferable: it helps to identify both macro- and microadenomas of the adrenal glands, as well as thickening of the adrenal pedicles, hyperplasia and other changes [14].
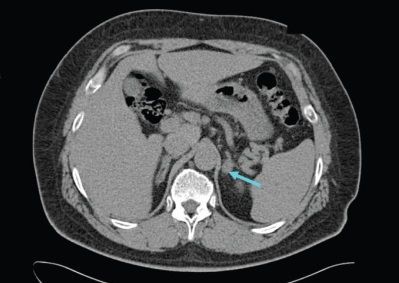
To clarify the form of hyperaldosteronism (unilateral and bilateral lesions), selective blood sampling is carried out from the veins of the adrenal glands in specialized centers [9]. This study effectively reduces the risk of unnecessary adrenal gland removal based on CT findings alone [4].
1.General information
Potassium is one of the key macroelements in the human body. This substance, especially in interaction with sodium, is necessary for the regulation of contractile activity of muscles (including the myocardium), water-salt, acid-base and energy balance, higher nervous activity, normal fermentation and metabolism, secretory activity of the endocrine glands, functioning liver and kidneys. Up to half of all salts in the body are potassium compounds, and most of them are contained in intracellular liquid spaces.
For all micro- and macroelements, including potassium, a certain concentration range must be observed.
Otherwise, both deficiency and excess lead to the development of severe, sometimes life-threatening conditions. Hyperkalemia, i.e. Excessive potassium levels are a typical example.
A must read! Help with treatment and hospitalization!
Calcium (Ca2+), Potassium (K+), Sodium (Na+), Chlorine (Cl-)
Calcium, potassium, sodium and chlorine
- These are the main electrolytes that ensure the maintenance of water and acid-base balance in the body. The presence of these elements in urine can provide information in the diagnosis of diseases accompanied by an imbalance in these balances and in monitoring their treatment. Calcium, potassium, sodium and chlorine ions ensure the maintenance of water and acid balance in the body. The amount of these electrolytes in 24-hour urine can provide valuable information for further diagnosis of diseases that arise from this imbalance and control of their treatment.
Calcium
Calcium plays a very important role in the human body. Calcium participates in physiological processes only in ionized form (participation in muscle contraction, hormone secretion mechanisms, receptor processes, cell division mechanisms, etc.).
The Ca++ concentration varies throughout the day: the minimum concentration level is observed at 20:00, and the maximum at 2–4:00 am. The level of ionized calcium is maintained by parathyroid hormone, calcitonin, and the active form of vitamin D3. The production of these hormones, in turn, depends on the level of Ca++. Its concentration in the blood is influenced by many factors - proteins, magnesium (it is necessary to study the concentration of magnesium if hypocalcemia is detected!). The acid-base status (ABS) is very important: alkalosis increases binding and reduces concentration; and acidosis, on the contrary, reduces binding and increases the concentration of ionized calcium in the blood.
Potassium (K+)
is the main cation of intracellular fluid.
Potassium (K+) is involved in the creation and maintenance of the electrical membrane potential of cells. Regulates intracellular osmotic pressure, stimulates the activity of glycolytic enzymes, participates in the metabolism of proteins and glycogen, plays an important role in the formation of the action potential in nerve and muscle cells and the conduction of nerve impulses, and has immunomodulatory activity.
The concentration of potassium in plasma (serum) depends on the balance of the following processes: potassium intake from the outside, distribution in the body and excretion (by the kidneys, sweat glands, through the intestines, etc.). There is no potassium depot in the body. Therefore, even with small changes in the concentration of potassium inside cells, its concentration in the plasma changes significantly. The uptake of potassium into cells is stimulated by insulin, and the uptake of potassium into cells is enhanced by the action of catecholamines and aldosterone. Changes in blood pH lead to changes in the K+ content in cells: during acidosis, it leaves the cells into the plasma; during alkalosis, it enters the cells. With hyperkalemia, ventricular tachycardia, ventricular fibrillation and even asystole are observed. With hypokalemia, muscle weakness, decreased reflexes, hypotension, disturbances in the conduction system of the heart, intestinal obstruction, and polyuria develop.
Sodium (Na+) is the main cation in the extracellular space
Sodium (Na+) is the most important osmotically active component of the extracellular space, which is associated with the regulation of the volume of extracellular fluid. 96% of the total sodium in the body is found outside cells. It is involved in the conduction of excitation in nerve and muscle cells, in the formation of an alkaline blood reserve and the transport of hydrogen ions.
The concentration of sodium in plasma (serum) depends on the balance of the following processes: sodium intake, its distribution in the body and excretion by the kidneys and sweat glands. The main regulators of sodium metabolism in the body are the renin-angiotensin-aldosterone system, ADH (vasopressin), and atrial natriuretic hormone.
Chlorine (Cl-) is the main anion of extracellular fluid and gastric juice
Chlorine ions play an important role in maintaining the acid-base state, osmotic pressure and water balance in the body. In biological media it is found predominantly in the state of the chloride anion Cl-.
Contained in plasma, lymph, cerebrospinal fluid. The balance of chlorine ions in the body is carried out by the presence of a balance between the processes of chlorine intake from food, distribution in the body and its excretion in urine, sweat and feces. A change in the concentration of sodium ions leads to a change in the concentration of the chloride anion. With the loss of chlorides, alkalosis develops; with excess consumption, acidosis develops.
Indications:
Calcium
- hyper- and hypocalcemia, especially in combination with dysproteinemia;
- studies of calcium status after citrated blood transfusions, heparin administration, major injuries, surgical interventions, sepsis, burns, pancreatitis, multiple organ failure, as well as patients with severe liver and kidney pathology, various malignant tumors, malabsorption;
- examination of pregnant women;
- sepsis;
- dialysis and extracorporeal circulation.
Potassium
- study of kidney function in their pathology;
- cardiovascular pathology;
- cardiac arrhythmias, arterial hypertension;
- adrenal insufficiency;
- monitoring the potassium content in the blood when prescribing diuretics and cardiac glycosides.
Sodium
- gastrointestinal disorders: vomiting, diarrhea;
- adrenal insufficiency;
- kidney diseases;
- dehydration, increased fluid loss.
Chlorine
- monitoring and dynamic observation of acid-base disorders in various diseases;
- kidney diseases;
- diabetes insipidus;
- pathology of the adrenal glands.
Preparation
It is recommended to donate blood in the morning, between 8 am and 12 pm. Blood is drawn on an empty stomach, after 6–8 hours of fasting. It is allowed to drink water without gas and sugar. On the eve of the examination, food overload should be avoided.
Interpretation of results
Calcium
Units of measurement: mmol/l. Reference values: 1.03–1.23 mmol/l.
Increasing values:
- primary hyperparathyroidism;
- ectopic tumors that produce parathyroid hormone;
- excess vitamin D intake;
- malignant tumors (an increase in ionized calcium can occur with normal values of total calcium) and metastases;
- acidosis;
- taking medications: hydrochlorothiazide (long-term use), lithium, androgens.
Reducing values:
- primary hypoparathyroidism, pseudohypoparathyroidism;
- vitamin D deficiency;
- sepsis;
- acute pancreatitis;
- renal failure;
- severe damage to skeletal muscles;
- hemodialysis with low calcium concentration in the dialysate;
- after blood transfusions containing calcium complexing anions (citrate);
- after extensive injuries, surgical interventions;
- burns;
- multiple organ failure;
- magnesium deficiency;
- alkalosis;
- hypernatremia;
- atrophic gastritis;
- substances that bind calcium (citrate, oxalate, EDTA, heparin);
- medications (anticonvulsants, danazol, foscarnet, furosemide - initial effect), alcohol.
Potassium
Units: mmol/l.
Increased potassium levels (hyperkalemia):
- excessive intake of potassium into the body: rapid infusion of potassium solutions;
- release of K+ from cells into the extracellular fluid: with massive hemolysis, rhabdomyolysis, tumor disintegration, severe tissue damage, deep burns, malignant hyperpyrexia, acidosis;
- decreased release of K+ by the kidneys: acute renal failure with oligo- and anuria, acidosis, end-stage chronic renal failure with oliguria, Addison's disease, pseudohypoaldosteronism, hypofunction of the renin-angiotensin-aldosterone system, shock conditions, tissue ischemia;
- decrease in the volume of extracellular fluid - dehydration;
- taking medications such as amiloride, spironolactone, triamterene, aminocaproic acid, antitumor agents, digoxin, non-steroidal anti-inflammatory drugs, trimethoprim-sulfamethoxazole.
Low potassium levels (hypokalemia):
- insufficient intake of potassium into the body: during chronic starvation; diet low in potassium;
- loss of potassium by the body with intestinal secretions with frequent vomiting, profuse diarrhea, intestinal villous adenoma, intestinal fistulas, suction of stomach contents through a nasogastric tube;
- loss of potassium in the urine due to renal tubular acidosis, renal tubular failure, Fanconi syndrome, Conn's syndrome (primary aldosteronism), secondary aldosteronism, Cushing's syndrome, osmotic diuresis (diabetes mellitus), alkalosis, administration of ACTH, cortisone, aldosterone;
- redistribution of potassium in the body (increased entry of potassium into cells): during treatment with glucose and insulin, familial periodic paralysis, alkalosis;
- sweat loss in cystic fibrosis;
- treatment of megaloblastic anemia with vitamin B12 or folic acid;
- hypothermia;
- taking corticosteroids, diuretics (except potassium-sparing), beta-blockers, antibiotics;
- administering large amounts of low-potassium fluid;
- VIPoma (tumor of pancreatic islet cells that secretes vasoactive intestinal polypeptide - VIP);
- magnesium deficiency.
Sodium
Units: mmol/l.
Increased sodium levels (hypernatremia):
- hypertensive dehydration: a) loss of fluid through the skin due to severe sweating; b) loss of fluid through the lungs during prolonged shortness of breath; c) loss of fluid through the gastrointestinal tract with frequent vomiting and severe diarrhea; d) with high fever (typhoid fever, paratyphoid fever, typhus, etc.);
- insufficient intake of water into the body;
- sodium retention in the kidneys (reduced urinary excretion) with primary and secondary hyperaldosteronism, Cushing's syndrome (excess of corticosteroids);
- excessive administration of sodium salts, for example, hypertonic sodium chloride solution;
- taking medications such as ACTH, anabolic steroids, androgens, corticosteroids, estrogens, methyldopa, oral contraceptives, sodium bicarbonate.
Low sodium levels (hyponatremia):
- insufficient sodium intake into the body;
- loss of sodium due to vomiting, diarrhea, severe sweating with adequate water and inadequate salt replacement;
- overdose of diuretics;
- adrenal insufficiency;
- acute renal failure (polyuric stage);
- osmotic diuresis;
- hypotonic overhydration: a) excessive parenteral fluid administration; b) reduced excretion of water in renal failure, increased secretion of vasopressin, deficiency of corticosteroids;
- dilution hyponatremia with edema and ascites in chronic heart failure, liver cirrhosis, liver failure, nephrotic syndrome;
- taking medications such as furosemide, aminoglycosides, hypertonic glucose solution, non-steroidal anti-inflammatory drugs, amitriptyline, haloperidol;
- hypothyroidism
Chlorine
Units: mmol/l.
Increased chlorine levels (hyperchloremia):
- dehydration due to insufficient water intake in the body;
- acute renal failure (when the consumption of chlorides exceeds their excretion during anuria, oliguria);
- diabetes insipidus;
- corticosteroid therapy;
- respiratory alkalosis;
- hyperfunction of the adrenal cortex.
Decreased chlorine levels (hypochloremia):
- increased sweating (with secretory dysfunctions and hormonal imbalance);
- overdose of diuretics;
- respiratory and metabolic acidosis;
- dehydration due to fluid loss due to vomiting, diarrhea;
- aldosteronism;
- polyuric stage of renal failure;
- head injuries;
- water intoxication with an increase in the volume of extracellular fluid;
- taking laxatives.