AST and ALT
Tests for AST and ALT are two studies that are often prescribed by a doctor in combination. Why is it so important to measure the levels of these two enzymes at the same time? The idea of the information content of their relationship was first put forward by scientist Fernando De Ritis from Italy. He used the method for the differential diagnosis of hepatitis of various types. Since then, his name has become a household name. The Ritis coefficient shows the ratio of AST and ALT activity. In healthy people, the coefficient is 0.91-1.75. The indicator is informative only if the values of these enzymes exceed the reference values. If in a double test only the AST values are exceeded, this means that the myocardium is damaged. When the heart muscle is damaged, the AST level increases 8-10 times, ALT - only 1.5-2 times. If a patient has a liver problem, on the contrary, the ALT level increases 8-10 times, and AST values only 2-4 times.
Indications for analysis
An analysis of AST levels is prescribed by a specialist in the following cases:
- Suspicion of heart pathology;
- Muscle dysfunction;
- To determine the condition of the liver and its capacity;
- Kidney pathologies;
- Diseases the appearance of which is caused by infections;
- Encephalopathy of unknown origin;
- Autoimmune pathologies;
- Jaundice and diseases associated with impaired bilirubin production;
- Chronic pancreatitis;
- Purulent-septic diseases;
- Gallbladder diseases;
- Diseases of the endocrine system;
- Oncological pathologies in a malignant form;
- Atypical reactions of the body to exposure to allergens, resulting in diseases of the epidermis;
- Muscle tissue injuries;
- Preparation for surgery;
- During therapy to assess the effectiveness of treatment.
An ALT test is prescribed if there is a suspicion of liver pathology. In this case, the following symptoms arise:
- Abdominal pain localized in the right hypochondrium;
- Change in color of the epidermis and whites of the eyes to a yellow tint;
- Bitterness in the mouth;
- Nausea accompanied by vomiting;
- Increased body temperature;
- The child gets tired quickly and eats poorly.
What is an enzyme
AST or AST is a protein synthesized inside the cells of the human body. Its highest concentrations are observed in the tissues of the myocardium, muscles and liver. To a lesser extent, the enzyme is present in the kidneys, pancreas, cells of the central nervous system and brain. It is encoded by the GOT 1 and GOT 2 genes. In a healthy person, the level of the enzyme is quite low. Its active release into the blood begins when the heart muscle ruptures, as well as liver destruction as a result of hepatitis, cirrhosis or cancer. The enzyme is important because it contains vitamin B6, which is involved in amino acid metabolism and, accordingly, the synthesis of insulin. In tests, the indicator is measured in units per liter of blood.
Reasons for increasing enzyme concentrations
An increase in ALT and AST in a child occurs in most cases when a pathological process develops in the body.
An increase in AST concentration occurs for the following reasons:
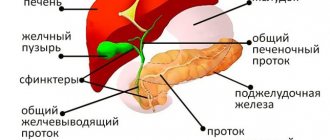
- Blockage of the bile ducts;
- Blood diseases;
- Pathologies of a rheumatological nature;
- Oncological pathologies in a malignant form;
- Heart diseases;
- Inflammation that occurs in muscle tissue;
- Intoxication of the liver and pathologies of this internal organ;
- Poor blood supply to the liver;
- Gastrointestinal diseases;
- Genetic pathology associated with poor copper metabolism;
- Thyroid diseases.
ALT concentration increases in the following situations:
- Almost all forms of hepatitis;
- Liver pathologies, including hereditary ones;
- Heavy metal poisoning;
- Oncological diseases of the liver of a malignant nature;
- Inflammation of soft muscle tissue;
- Heart pathologies;
- Blockage of the bile ducts;
- Muscular dystrophy.

An increase in AST and ALT levels also occurs not due to diseases. The reasons for this may be:
- Violation of the rules for submitting biomaterial for research;
- Postoperative period;
- Use of certain medications.
Range of application of AST analysis
- In cardiology as a marker of myocardial infarction. In the heart muscle the enzyme is more than 10,000 times more active than in the blood. During a heart attack, intense release of the enzyme occurs.
- For liver pathologies. Diseases such as hepatitis and cirrhosis are certainly accompanied by the destruction of liver tissue and a sharp jump in AST values.
- For chronic alcoholism.
- In obstetrics and gynecology. During pregnancy, a woman may experience a slight increase in AST values. This is explained by the effect of the growing fetus on the mother's liver. In the first trimester, its values should not exceed 31 U/l, in the second and third – 30 U/l.
- In endocrinology for diabetes and/or excess weight.
Enzyme standards for children
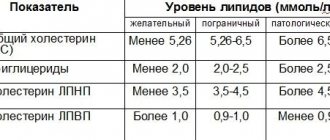
Each laboratory has its own reference values for this enzyme. Usually they focus on two main units of measurement of protein content in the body - mkat/l and U/l.
In this regard, reference protein values in children will be different.

Table 1. AST norm in children in mkat/l
| Age | Norm, mkat/l |
| Up to 1 month | 0,38-1,21 |
| From 1 to 12 months | 0,27-0,97 |
| From one year to 16 years | 0,10-0,63 |
Table 2. AST norm in children in U/l
| Age | Norm, Units/l |
| Up to 1 month | Up to 73 |
| From 1 to 12 months | Up to 58 |
| From one year to 16 years | Up to 38 |
Based on these reference values, the analysis is deciphered.
ALT also has its own reference values.
Table 3. ALT norm in children
| Age | Norm, units/l |
| Up to 12 months | Up to 60 |
| From 1 year to 3 years | 40-45 |
| From 3 to 6 years | |
| From 6 to 12 years |
How to prepare for analysis
To increase the reliability of the analysis, some rules and restrictions should be observed. So on the eve of the study, you should refrain from eating fatty and smoked foods, as well as confectionery. It is best if the test is performed on an empty stomach in the morning. When taking medications, you should consult your doctor about their possible withdrawal. The fact is that during a biochemical blood test, many medications can affect the results of the study. When treated with antidepressants, antibiotics, diuretics and other drugs, the test readings may be distorted. In addition, immediately before a visit to the treatment room, it is prohibited to perform ultrasound, x-ray examination and physiotherapy procedures.
How the research is carried out
The analysis is carried out in laboratory conditions. In older children, biomaterial is taken from a vein, in younger children - from the heel. There are no special rules for testing for infants. Older children are advised to adhere to the following recommendations:
- The biomaterial is taken in the morning on an empty stomach;
- Before the study, the child should be provided with comfortable psychological conditions and reduced physical activity;
- A few days before the analysis, fatty, spicy, smoked and salty foods are removed from the diet;
- A few days before the delivery of the biomaterial, they stop giving the child medications, and if it is not possible to interrupt therapy, a list of medications is presented to the doctor or specialist who will conduct the analysis.
Decoding the analysis for AST
Normal for women
AST test values vary depending on age and gender. So in women the norm of the enzyme in the blood is 31 U/l. The older a person is, the lower his activity in the body. This is due to a slowdown in metabolism in general. A natural physiological state such as pregnancy also affects enzyme levels. During this period, there may be small jumps in both one and the other direction.
Normal for men
The concentration of the enzyme in men begins to exceed its amount in women starting from 12-17 years. Its higher concentrations are explained by the large volume of muscle tissue. At puberty, the AST norm in boys is about 29 U/l. This is about four units higher than girls at the same age. In an adult man, the enzyme level can reach 37 U/l.
Hepatoprotective therapy for liver diseases in children
Non-alcoholic fatty liver disease (NAFLD) is a pressing problem in modern pediatrics. It should be noted that in recent years there has been an increase in the number of children suffering from NAFLD. Moreover, in 23–53% of cases, the development of NAFLD in children is associated with overweight or obesity [1–3].
NAFLD is represented by two clinical forms: fatty liver (steatohepatosis), non-alcoholic steatohepatitis (NASH). Non-alcoholic steatohepatitis is a chronic liver disease with histological signs of alcoholic hepatitis in individuals who do not drink alcohol in significant hepatotoxic doses [2–5].
According to modern concepts, primary and secondary NAFLD are distinguished. Primary NAFLD most often develops in the presence of type 2 diabetes mellitus, obesity and hyperlipidemia, and may be a manifestation of metabolic syndrome [6].
The causes of secondary hepatic steatosis and NASH in children may be [7]:
- medications (glucocorticoids, synthetic estrogens, antitumor, antibacterial, non-steroidal anti-inflammatory drugs, etc.);
- malabsorption syndrome due to surgical intervention (as a consequence of ileojejunal anastomosis, biliary-pancreatic stoma, extended resection of the small intestine, etc.);
- chronic diseases of the gastrointestinal tract accompanied by malabsorption syndrome (chronic pancreatitis, nonspecific ulcerative colitis);
- long-term (more than 2 weeks) parenteral nutrition;
- rapid weight loss;
- abetalipoproteinemia, limb lipodystrophy, Weber-Christian disease, Wilson-Konovalov disease;
- bacterial overgrowth syndrome.
It should be noted that with NAFLD in children, in 48–100% of cases there may be no symptoms characteristic of liver pathology. In this case, 20–30% may experience vague discomfort, heaviness, aching pain in the right hypochondrium, and asthenic syndrome. Some children complain of burping and heartburn, which is most often associated with obesity. Hepatomegaly can be detected in 75% of cases.
Increased levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are usually observed in the blood. In this case, the AST/ALT ratio is usually below 1, and an increase in this ratio is observed with the development of liver fibrosis. In addition, there may be a slight increase in alkaline phosphatase (ALP) and gammaglutamyltransferase up to two norms, hyperbilirubinemia up to 1.5–2 norms and dyslipidemia. Hypoalbuminemia and prolongation of prothrombin time may indicate the development of liver cirrhosis [5, 8, 9].
Ultrasound examination reveals distal attenuation of the echo signal, diffuse hyperechogenicity of the liver (“bright liver”), increased echogenicity of the liver compared to the kidneys, and blurred vascular pattern.
Carrying out a computed tomography scan for NAFLD can reveal a decrease in the radiodensity of the liver to 3–5 units (with a norm of 50–75 units). In this case, the radiodensity of the liver is less than the radiodensity of the spleen. Visualization of intrahepatic vessels, portal and inferior vena cava as denser structures compared to liver tissue. Focal fatty degeneration is characterized by the intersection of normal liver blood vessels in zones of reduced radiocontrast [8, 9].
The main histological signs of NASH include: 1) fatty degeneration of hepatocytes (large and small droplets); 2) mixed inflammatory infiltration (neutrophils, lymphocytes, macrophages); 3) fibrosis (mainly perivenular); 4) additional (non-permanent) signs - Mallory bodies, focal centrilobular necrosis, iron deposits [8, 9]. Before talking about NAFLD, it is necessary to conduct a comprehensive examination of the child [5].
When NAFLD is confirmed in a child, it is important to select an effective and safe treatment method to prevent the progression of the disease and treat existing disorders.
The main goal of therapy for NAFLD is to prevent progression of the disease and the development of cirrhosis. Currently, there is no uniform standard of treatment for patients with NAFLD. The main directions of therapy: correction of obesity and insulin resistance; reduction of lipid peroxidation and oxidative stress (Table 1).
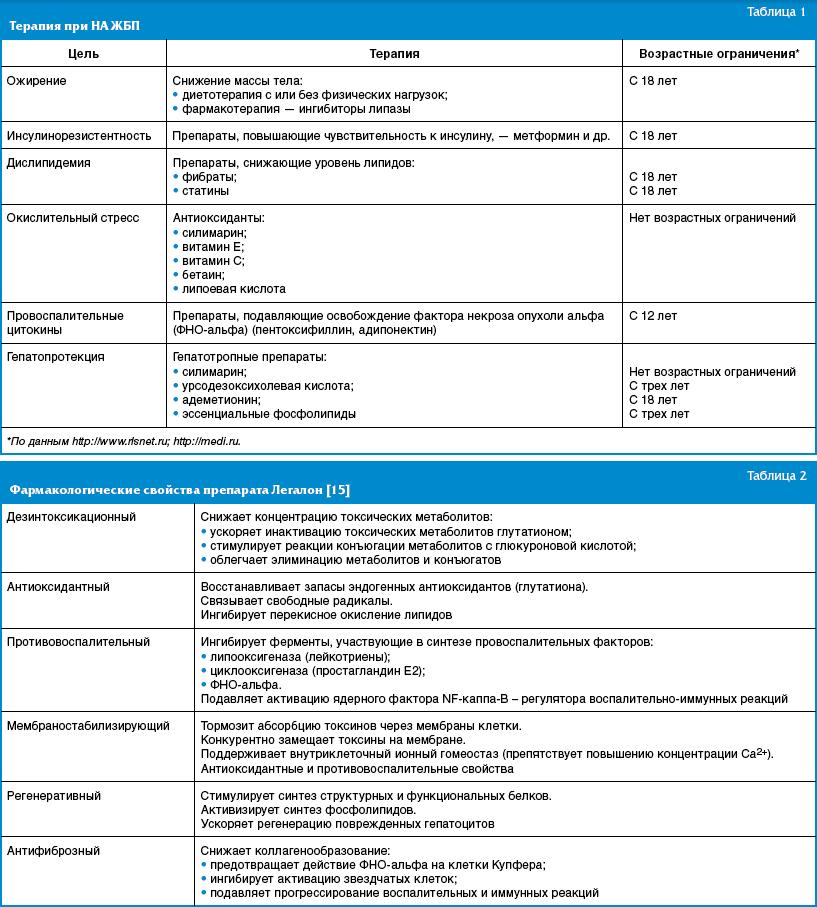
The most important thing in the treatment of NAFLD is weight loss. At the same time, sudden weight loss is not recommended, which can contribute to increased inflammation and the formation of liver fibrosis. It should be noted that some drugs approved for the treatment of adult patients are not registered for use in pediatrics, and some approved drugs are not sufficiently effective.
For example, in Russia orlistat is not approved for use in children. Metformin, which in foreign practice has been used since the age of 10 for metabolic syndrome and type 2 diabetes, is not always effective. According to recent studies, a 6-month course of therapy with this drug improved the metabolic profile, but had no effect on the histological picture of the liver [5].
A fairly effective drug that is used for NAFLD, inhibits the progression of the disease and has an antifibrotic effect, is the standardized silymarin - Legalon. Legalon is a medicine made from herbal raw materials. The active substance is isolated from the fruits and milky juice of milk thistle (milk thistle). Milk thistle has been used as a medicine for thousands of years, and it was only in 1968 that the biochemical composition of milk thistle was deciphered at the Munich Pharmaceutical Institute. The main component of the medicinal plant milk thistle are flavonoids with hepatoprotective properties: silibinin, silydianin, silicristin (the general name for a mixture of these compounds is silymarin). Silymarin extract is water soluble and poorly absorbed in the intestines. To increase the bioavailability of the active substance of the drug Legalon, a patented co-precipitation process is used, in which the bioavailability of the reference silymarin increases to 85% (the bioavailability of other silymarins is no more than 20%). In all countries there is an international patent for the galenic form of standardized silymarin, which increases the absorption of the active substance.
It has been proven that the effectiveness and safety of silymarin depends on the ratio of flavolignans in the drug and the dose of silibinin. The difference in composition leads to changes in pharmacokinetics and, consequently, clinical effectiveness in the treatment of liver diseases of various etiologies.
An analysis of silymarin-containing drugs (high-performance liquid chromatography profile) found that in terms of the total content of biologically active substances (flavolignans), Legalon takes first place, and Karsil, which contains 5 times less flavonolignans than Legalon, takes last place.
The drug Legalon, compared to other drugs, contains a higher content of silicristin and silibinin [15].
Silibinin is the main pharmacologically active substance. Numerous experimental and clinical studies have made it possible to clarify the mechanism of action and clinical effectiveness of silymarin in acute and chronic liver diseases. As a result, it was found that the reference silibinin (Legalon) has antifibrotic, antioxidant, antitoxic, cytoprotective, anti-inflammatory, immunomodulatory, and antitumor activity (Table 2) [10, 11].
Legalon is recommended for the treatment of toxic and medicinal liver damage.
In Western Europe, acute drug-induced hepatitis accounts for 15–20% of fulminant hepatitis, in Japan - 10%, in Russia - 5%. In etiological terms, anti-tuberculosis and antibacterial drugs are in first place, then non-steroidal anti-inflammatory drugs, drugs that regulate the functions of the nervous system, hormonal, cytostatic, hypotensive, antiarrhythmic drugs.
The pathological process begins with the action of hepatotoxicants - steatosis, necrosis, cholestasis, fibrosis (cirrhosis). During biotransformation, the drug substance does not sufficiently lose its toxicity and/or during the metabolism of the initially non-toxic compound, intermediate or final hepatotoxic metabolites appear. The disease occurs when there is an overdose of drugs, a deficiency of conjugation substrates, coenzymes and enzymes necessary for detoxification. In this case, a toxic substance can directly affect the structure of the hepatocyte (for example, the toxic metabolite of paracetamol - N-acetyl-p-benzoquinone) and/or have an indirect effect on specific metabolic reactions (for example, inhibition of protein synthesis when using cytostatic antibiotics). The classic drug with an obligate hepatotoxic effect is paracetamol. On the other hand, liver damage may be caused by genetically determined metabolic characteristics or increased susceptibility of the body to a drug that is not a hepatotoxicant. This type of pathology is not reproduced experimentally and is not dose-dependent in nature [12].
In children, liver damage is most often observed as acute hepatitis (in approximately 80% of cases). Chronic drug-induced liver damage can be an independent disease, but more often develops as an outcome of an acute hepatotoxic pathological process (with prolonged use of drugs). The severity of drug-induced liver diseases varies from asymptomatic elevation of transaminases to the development of fulminant liver failure. In children, asymptomatic increases in transaminases are more common. Less commonly, with serious liver damage, jaundice, itching, “liver signs”, bleeding, liver enlargement and pain on palpation may be observed. With immune-mediated damage, myalgia and arthralgia, conjunctivitis, rhinitis, skin rash, lymphadenopathy, and fever can be observed. Drug-induced liver damage can occur within 48 hours while taking medications. In some cases, the disease may take several weeks or months to appear. Some drug reactions are transient and resolve spontaneously.
Depending on the degree of increase in the levels of ALT and alkaline phosphatase, acute liver damage is classified as hepatocellular (cytolytic), cholestatic or mixed, combining signs of cholestasis and cytolysis (Table 3).
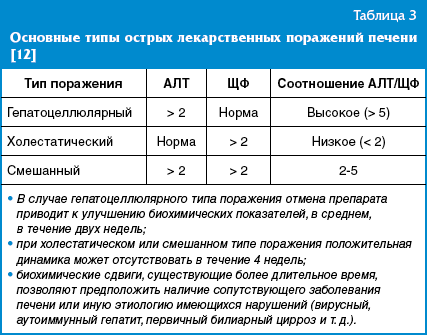
In childhood, the hepatocellular type of damage is more common (in 2/3 of cases).
Elevated ALT is the most sensitive test for early diagnosis of drug-induced hepatitis. In mitochondrial hepatocytopathies, aspartate aminotransferase (AST) activity may also increase. When the de Ritis ratio (AST/ALT) is less than 1, the increase in transaminases is interpreted as an inflammatory type of response, more than 1 - as a necrotic type.
A feature of cholestatic forms of drug-induced liver damage is the frequent absence of hypertransaminasemia. In this case, jaundice and itching of the skin develop, but overall health, as a rule, does not suffer.
Typically, hepatoprotectors are used to prevent and treat drug-induced hepatitis. Considering the mechanisms of action, Legalon is the drug of choice for drug-induced hepatitis.
In pediatrics, the following dose selection scheme for Legalon is used: standardized silymarin - 5 mg/kg/day (3 times a day with food, water). Children up to 40 kg - 70 mg/day (1 capsule of Legalon 70 mg) per day; 41–60 kg - 240 mg/day (2 capsules of Legalon 70 mg per day); 61–70 kg - 320 mg/day (3 capsules of Legalon, 70 mg per day) [14].
Clinical observation
Children with NAFLD were observed in the Department of Gastroenterology of the Moscow Research Institute of Pediatrics and Pediatric Surgery - 9 children (6 boys and 3 girls) aged 11 to 15 years. Upon admission, 4 children (44%) had periodic abdominal pain, 3 (33%) had heartburn, and 5 (56%) had belching. Obesity was observed in 7 children (78%). Moreover, the average body mass index (BMI) in this group of children was 97.5 ± 1.2 percentile.
All children were carefully examined to clarify the nature of liver damage. As a result of the examination, steatohepatitis was detected in 7 children; steatohepatosis was observed in two children against the background of hyperlipidemia. Moreover, all children with steatohepatitis had an increase in the level of ALT in the blood (105 ± 19.5 U/l) and only 5 (71%) had an increase in the level of AST (75.2 ± 11.4 U/l). In 4 (57%) children there was an increase in alkaline phosphatase and in 1 (14%) there was an increase in the level of gamma-glutamyl transpeptidase (GGTP) within 1.5 norms. The level of total bilirubin was slightly increased in 3 children (43%).
One child with steatohepatosis had an increase in blood cholesterol levels, and the second had an increase in cholesterol and triglycerides. Ultrasound examination revealed signs of steatosis in all children: distal attenuation of the echo signal, diffuse hyperechogenicity of the liver, and unclear vascular pattern. Moreover, 5 (56%) of them had hepatomegaly.
All children were prescribed diet therapy and Legalon at an age-specific dosage of 5 to 10 mg/kg/day (1–3 capsules per day) for 1.5 months. The dynamics of clinical (improvement of general condition, reduction of dyspeptic disorders, liver size), biochemical (normalization of ALT, AST, GGTP levels) and ultrasound (reduction of liver size, improvement of echostructure) indicators were considered as criteria for effectiveness.
When examining patients after 1.5 months, abdominal pain persisted in 2 children (22%), heartburn in 1 child (11%), and belching in 2 children (22%). Body weight decreased slightly in 4 obese children (57%); in 3 children (43%) body weight did not change. Moreover, the average BMI in this group of children was 96.6 ± 1.0 percentile. When studying biochemical parameters, a significant decrease in the activity of serum transaminases was revealed (ALT - 51.1 ± 18.2 U/l, AST - 43.5 ± 6.6 U/l). At the same time, in 5 children (56%) the ALT level returned to normal, and in 2 (22%) it decreased. The AST level normalized in 3 children (33%) and decreased in 2 (22%). GGTP levels after treatment were normal in all children. ALP decreased to normal levels in 2 children, bilirubin - in one child. In the remaining children, these indicators (alkaline phosphatase and bilirubin) did not change significantly.
As for children with hyperlipidemia, one patient had normal cholesterol levels after treatment, and the second had normalized triglyceride levels.
Moreover, during treatment with Legalon, a positive dynamics of the ultrasound picture was observed in the form of a decrease in the size of the liver in 3 out of 5 children (60%), and in 5 out of 9 children (56%) an improvement in its echogenicity was detected.
Thus, Legalon is an effective hepatoprotective drug that helps to significantly reduce transaminases in the blood and improve the ultrasound picture of the liver in children with NAFLD.
Due to the pronounced positive dynamics, therapy, including diet and Legalon, was continued for another 3 months with the appointment of a subsequent control blood test and ultrasound of the abdominal cavity.
Also in our department, 4 children with drug-induced hepatitis were observed, three of them received long-term anticonvulsant therapy and one child received paracetamol for three days, then he was prescribed antibacterial therapy for 10 days. During therapy, children experienced an asymptomatic increase in transaminases in the blood. At the same time, all four had an increase in the level of ALT in the blood (104.3 ± 14.1 U/l), and two had an increase in AST (87.5 ± 11.5 U/l). The rest of the indicators were normal.
In this regard, all children were prescribed Legalon as a hepatoprotective agent at an age-specific dosage of 5 mg/kg/day during the main treatment.
In a child receiving paracetamol and antibacterial therapy, an increase in transaminases (ALT and AST) was detected on the second day of antibacterial therapy (drug-induced hepatitis could be associated with both paracetamol and antibacterial therapy), and hepatoprotective therapy was prescribed on the same day with the drug Legalon. After 2 weeks (one week after finishing taking the antibiotic), a repeat biochemical analysis showed the level of transaminases in the blood to be normal. Legalon contributed to a faster recovery of liver function.
As for children receiving anticonvulsant therapy, after a month while taking Legalon, transaminase levels completely returned to normal. For preventive purposes, these children continued to receive Legalon against the background of anticonvulsant therapy and after normalization of indicators.
Thus, standardized silymarin (Legalon) is a rational hepatoprotective drug that is recommended for use in children with various liver diseases for preventive and therapeutic purposes. The duration of therapy depends on the severity of the disease and lasts from a month or more.
Literature
- Tominaga K., Kurata JH, Chen YK et al. Prevalence of fatty liver in Japanese children and relationships to obesity: an epidemiological ultrasonographic survey // Dig Dis Sci. 1995; 40: 2002–2009.
- Schwimmer JB, Deutsch RK, Kahen T. et al. Prevalence of fatty liver in children and adolescents//Pediatrics. 2006; 118:1388–1393.
- Arslan N.B., Buyukgebiz B., Ozturk Y. et al. Fatty liver in obese children: prevalence and correlation with anthropometric measurements and hyperlipidemia // Turk J Pediatr. 2005; 47:23–27.
- Paul Angulo. Nonalcoholic Fatty Liver Disease // N Engl J Med. 2002; 346:1221–1231.
- Kaganov B. S. Children's hepatology. M.: Dynasty Publishing House, 2009. 576 p.
- Burt AD, Mutton A., Day CP Diagnosis and interpretation of steatosis and steatohepatitis // Semin Disgn Pathol. 1998; 15: 246–258.
- Hassink SG A Clinical Guide to Pediatric Weight Management and Obesity. Lippincott Wiliams and Wilkins, 2007; 114–129.
- Pinto HC, Baptista A., Camilo ME, Valente A., Saragoca A., de Moura MC Nonalcoholic steatohepatitis: clinicalopathological comparison with alcoholic hepatitis in ambulatory and hospitalized patients // Dig Dis Sci. 1996; 41: 172–179.
- Perrie E., Pardee B.S., Joel E. Lavine, Jeffrey B. Schwimmer. Diagnosis and Treatment of Pediatric Nonalcoholic Steatohepatitis and the Implications for Bariatric Surgery. Seminars in Pediatric Surgery. Volume 18, Issue 3, Pages 144–151, August 2009.
- Saller R., Meier R., Brignoli R. The use of silymarin in the treatment of liver diseases // Drugs. 2001; 61:2035–2063.
- Butorova L. I., Tsibizova T. A., Kalinin A. V. Possibilities of using Legalon for non-alcoholic fatty liver disease // Experimental and clinical gastroenterology. 2010. No. 5. pp. 69–75.
- Butorova L. I., Kalinin A. V., Loginov A. F. Drug-induced liver damage. Educational and methodological manual for doctors. M., 2010. 64 p.
- DeLeve LD, Kaplowitz N. Mechanisms of drug-induced liver disease // Gastroenterol Clin N Am. 1995; 24: 787–810.
- Ladas EJ, Kroll DJ, Oberlies NH et al. A randomized, controlled, double-blind, pilot study of milk thistle for. the treatment of hepatotoxicity in childhood acute lymphoblastic leukemia (ALL) // Cancer. 2010, Jan 15; 116(2):506–513.
- Matveev A.V., Konyaeva E.I., Kurchenko V.P., Shchekatikhina A.S. Hepatoprotective properties of silymarin // Clinical pharmacology. 2011, No. 2.
M. L. Babayan, Candidate of Medical Sciences A. I. Khavkin, Doctor of Medical Sciences, Professor
Federal State Budgetary Institution "Moscow Research Institute of Pediatrics and Pediatric Surgery" of the Ministry of Health of the Russian Federation, Moscow
Contact information for authors for correspondence
Diagnosis and treatment
If the concentration of enzymes is increased, it is important to establish the reason for the deviation of AST and ALT from normal.
This can only be done by a specialist based on the results of some studies. Diagnosis is not made using a biochemical blood test alone. The doctor prescribes the following diagnostic measures:
- CT scan;
- General blood analysis;
- Ultrasound and ECG;
- Liver biopsy if there is a suspicion of pathology at this internal level;
- Analysis of iron levels in the blood.
When determining the cause of elevated enzyme concentrations, it is also important to look at bilirubin, alkaline phosphatase, and GGTP levels.
If there is a suspicion of a malignant oncological pathology, tests for tumor markers and other tests are prescribed to help diagnose cancer.

Only after determining the reasons for the increase in the concentration of enzymes in the blood, appropriate therapy is prescribed. If the treatment gives a positive result, the protein level returns to normal.