Patient K, 69 years old.
Diagnosis
: left kidney tumor T1aNoMx. Left kidney cyst.
Clinical manifestations of the disease:
pain in the lumbar region on the left.
History of present illness:
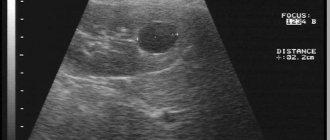
In 1998, at the place of residence, an ultrasound revealed a mass in the left kidney. She was examined periodically and an increase in size was noted.
According to multislice computed tomography, in the middle segment of the left kidney on the posterior surface, an oval-shaped formation of a heterogeneous structure, 25x12 mm in size, is determined, actively accumulating a contrast agent; A cyst measuring 65x66 mm is closely adjacent to the formation.
Hospitalized at the Urology Clinic of the First Moscow State Medical University named after. THEM. Sechenov for further examination and surgical treatment.
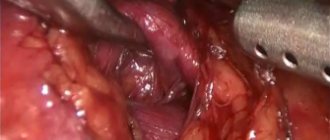
In order to rid the patient of the tumor of the left kidney, to prevent the progression of the tumor process, as well as to rid the patient of the left kidney cyst, laparoscopic (retroperitoneoscopic) resection of the left kidney with the tumor and resection of the walls of the left kidney cyst were performed.
VIDEO
Video description:
the operation is performed without clamping the renal vessels (under conditions of zero ischemia). No safety drain was installed. The duration of the operation is 40 minutes. She was not in the intensive care unit. The postoperative period was uneventful. On the third day the patient was discharged home in satisfactory condition. The sutures were removed on the seventh day on an outpatient basis.
Histological conclusion:
in preparations, clear cell renal cell carcinoma (G1) with invasion into the renal cortex. The surgical margin is negative.
What is partial nephrectomy?
Kidney resection (from the Latin resectio - cutting off) - removal of part of the kidney (other names for this operation: partial nephrectomy, partial nephrectomy) affected by some pathological process (tumor, less often - stones, cysts, injuries, tuberculosis, etc. ), within healthy tissue (i.e. the tumor is removed as much as possible, while the surgeon goes a little into healthy tissue).
Today, classical open resection has faded into the background; its modern minimally invasive alternative, laparoscopic resection, has taken the leading position, which will be discussed further, namely, indications, contraindications, technique, etc.
Cost of laparoscopic kidney resection
The operation is performed under a compulsory medical insurance policy or according to a quota, depending on the volume and type of operation.
Who is indicated for laparoscopic partial nephrectomy?
Kidney resection is most effective for localized cancer (the tumor does not grow into neighboring organs or the kidney capsule), a small tumor size (no more than 4 cm) and a single tumor node. Most often, the operation is performed at stages T1 and T2. With such tumor parameters, the risk of relapse after surgery is extremely small and is less than 10%.
In addition to the above, partial nephrectomy is performed in cases where kidney removal would leave the patient without both kidneys or would require immediate initiation of dialysis.
This situation is possible when:
- bilateral kidney cancer (if both kidneys are affected by the tumor),
- cancer of a single kidney (congenital absence of a kidney or after surgical removal),
- a single functioning kidney (i.e., despite the presence of both kidneys, one of them has lost its function for some reason),
- in case of diseases of the kidney (which is not affected by a tumor) that potentially threaten its function (i.e. if on one side the kidney is affected by a tumor, and on the opposite side there is a disease that subsequently can or will lead to loss of its function).
Laparoscopic nephrectomy
There are a fairly large number of diseases for which routine kidney removal may be indicated. The most common of them are kidney cancer, hydronephrosis and urolithiasis. Malignant tumors of large size (usually more than 5 cm), or located deep in the kidney, near large vessels, do not allow removal of the tumor while preserving the organ. Hydronephrosis is a disease that develops as a result of long-term disruption of the outflow of urine from the kidney. As a result, the kidney stops secreting urine, and its parenchyma becomes thinner. Such an organ does not perform any function, but it can be a source of chronic inflammation, cause pain, and increased blood pressure. This is advanced urolithiasis, when chronic inflammation in the kidney leads to its shrinkage.
What are the contraindications to laparoscopic partial nephrectomy?
Like all laparoscopic operations, kidney resection has a general list of contraindications, in which the operation is associated with a high risk of complications such as bleeding, or surgical intervention will be difficult.
Resection is contraindicated in:
- extreme obesity,
- disruption of the blood coagulation system,
- for infectious diseases,
- in late pregnancy.
Acute inflammatory processes due to the high likelihood of infection of the abdominal cavity are also a contraindication to laparoscopic resection. The presence of scars in the peritoneal cavity from previously performed operations causes significant technical difficulties in isolating the kidney and its surrounding tissues, which significantly complicates or makes laparoscopic intervention impossible.
Classification
Currently, in Russia and in most countries of the world, the TNM classification (T-tumor, N-node, M-metastasis) is used.
- T—primary tumor TX —primary tumor cannot be assessed
- T0 - no data on the primary tumor
- T1 - tumor no more than 7 cm in greatest dimension, limited to the kidney
- T1a - tumor up to 4 cm
- T1b - tumor 4-7 cm
- T2 - tumor more than 7 cm in greatest dimension, limited to the kidney
- T3 - the tumor spreads into large veins, or invades the adrenal gland or surrounding tissues, but does not extend beyond the Gerota's fascia
- T3a - tumor invasion of the adrenal gland or perirenal tissue within Gerota's fascia
- T3b - Tumor extends into the renal vein or inferior vena cava below the diaphragm
- T3c - the tumor extends into the inferior vena cava above the diaphragm or invades its wall.
- T4 - Tumor extends beyond Gerota's fascia
- N - regional lymph nodes NX - regional lymph nodes cannot be assessed
- N0 - no metastases in regional lymph nodes
- N1 - metastasis in one regional lymph node
- N2 - metastases in more than one regional lymph node
- M - distant metastases MX - distant metastases cannot be assessed
- M0 - no distant metastases
- M1 - distant metastases
- G - histopathological grading GX - degree of differentiation cannot be assessed
- G1 - well differentiated tumor
- G2 - moderately differentiated tumor
- G3-4 - poorly differentiated/undifferentiated tumor
Grouping by stages
| Stage I | T1 | N0 | M0 |
| Stage II | T2 | N0 | M0 |
| Stage III | T1 | N1 | M0 |
| T2 | N1 | M0 | |
| T3 | N0, N1 | M0 | |
| Stage IV | T4 | N0, N1 | M0 |
| any T | N2 | M0 | |
| any T | any N | M1 |
How to prepare for surgery?
Before the operation, you must be consulted by specialists of various profiles - an anesthesiologist, therapist, surgeon, and, if necessary, others. After this, your attending physician gives individual recommendations that you must strictly follow - do not eat, do not drink several hours before surgery, etc.
Be sure to disclose any drug allergies or medications you are taking to avoid complications during surgery (especially blood thinners, which can cause serious bleeding both during and after surgery).
Before the operation, a thorough bowel preparation is carried out, and broad-spectrum antimicrobial drugs are prescribed to prevent infectious complications in the postoperative period.
How is laparoscopic partial nephrectomy performed?
The operation is performed under general anesthesia (you will be put to sleep throughout the entire operation). The patient is placed in a special position - on the healthy side, while the operating table is extended, thereby bringing the kidney closer to the anterior abdominal wall. Access to the kidney is carried out using 3-4 small holes of about 1 cm on the anterior abdominal wall, through which a special camera (laparoscope) and other necessary surgical instruments are inserted. One of the holes is subsequently extended slightly so that the bag with the tumor can be removed from the abdominal cavity.
The laparoscope increases the size of the surgical field several times, which allows the surgeon to work more accurately and accurately. This is necessary during kidney resection, since resection, unlike kidney removal, is a technically more complex manipulation that requires more precise and careful actions. To increase the working space in the abdominal cavity, carbon dioxide is introduced into it (creating pneumoperitoneum), which is completely removed at the end of the operation.
After creating pneumoperitoneum, a laparoscope is inserted and the abdominal cavity is examined. Next, the surgeon begins to isolate the kidney; to do this, he dissects the tissue and moves the organs to the sides. After isolating and examining the kidney, the stage of isolating the vessels and ureter begins, then the surgeon clamps the renal artery with a special clamp to ensure minimal blood loss during the operation.
After this, the most critical stage begins - the resection itself, which is performed under conditions of kidney ischemia (as mentioned earlier, the renal artery is pinched, therefore, arterial blood does not flow to the kidney, which can lead to its death if the ischemia time is more than 40 minutes) . On average, the ischemia time is about 10–15 minutes, which is absolutely safe for the kidney and does not affect its functions in any way.
After removing the area of the kidney with the tumor, the edges of the kidney are sutured and the clamp is removed from the artery. The abdominal cavity and the bed of the removed tumor are examined for bleeding, the tumor is placed in a special bag and removed from the abdominal cavity. The surgeon then removes the instruments and stitches the tissues together. On average, the duration of the operation varies from 2 to 3 hours.
The incidence of renal cell carcinoma (RCC) has been steadily increasing in recent years. Currently, the increase in the incidence rate is about 3% annually. At the same time, the greatest increase is observed due to an increase in the number of patients with localized tumors, which is due to improved diagnostic methods [2, 11]. Thus, according to the European SEER (Surveillance, Epidemiology, and End Results) database, from 1988 to 2002, the average size of a kidney tumor decreased from 67 to 59 mm. When analyzing a large cohort of patients operated on in European countries, it was revealed that during the observation period in 1995-2005. the number of surgical interventions performed for RCC increased from 6.2 to 7.5 per 100,000 patients, while the proportion of T1 tumors increased from 36.6 to 44.2%, and the proportion of advanced RCC decreased from 46.4 to 33 .7% [10]. The number of patients with a tumor less than 4 cm in diameter increased from 30 to 39%. The identified trends undoubtedly led to improved survival rates in these RCC groups [11]. Due to stage migration towards localized RCC with small tumors, there has been a gradual change in the primary surgical treatment modality in this group of patients over the past 2 decades. Radical nephrectomy, previously considered the “golden” standard for the treatment of localized stage T1-2 RCC, has been replaced by organ-sparing kidney resection in various access options: standard, laparoscopic or robot-assisted. Laparoscopic partial nephrectomy (LRP) has recently become the standard of care in clinics with significant experience in laparoscopic surgery for small exophytic kidney tumors (less than 2.5-3.0 cm). The main disadvantages of LRP are the difficulties in ensuring reliable intraoperative hemostasis and the need to create ischemia of the renal tissue.
This paper presents the interim results of using a new LRP technique using radiofrequency ablation (RFA) without ischemia in patients with early RCC.
Materials and methods
We used the equipment and a standard set of instruments necessary to perform laparoscopic operations, including a laparoscopic stand, insufflator, video camera, video monitor, light guide, light source, apparatus for monopolar and bipolar coagulation, apparatus for bipolar coagulation "LigaShu", irrigation and suction device, laparoscopic Ultrasound sensor. Instrumentation: Veress needle, disposable trocars of various sizes, cutting-coagulating scissors, clamps, dissectors, needle holders, laparoscopic vascular clamp, fan-shaped retractor, clipper, electrodes for mono- and bipolar coagulation, a set of atraumatic suture material, laparoscopic container for drug removal. For LRP using RFA, a monopolar Cool-Tip RF unit with a single-needle probe (17 G., length 20 cm, working surface 20 mm) and a set of passive electrodes was used.
Before surgery, all patients underwent ultrasound (US) and computed tomography (CT) of the abdominal organs and retroperitoneal space, with the help of which the exact location of the tumor, its relationship to the renal vessels and the collecting-pelvic system (PSS) were assessed.
The patient's position on the operating table was on the side (opposite to the kidney in which the tumor was localized), a cushion was placed at the level of the xiphoid process. The operation was performed through a transabdominal approach. After creating pneumoperitoneum, 3 trocars were installed in the abdominal cavity (+1 for a retractor if traction of the liver is necessary): I - 10 mm trocar - in the periumbilical region (along the lateral edge of the rectus abdominis muscle), II - 10 mm trocar - along the midclavicular line below costal arch, III - 10 mm trocar - along the midclavicular line above the anterosuperior iliac spine.
A laparoscope was inserted into port I and a video inspection of the abdominal cavity was performed, and instruments were placed into ports II and III.
The stages and methodology of standard laparoscopic and open partial nephrectomy are not fundamentally different. After revision of the abdominal cavity, mobilization of the kidney and renal vessels was performed. Then, using mono- and bipolar coagulation, the perinephric tissue around the tumor was dissected. Fiber was left directly above the tumor. In the case of intraparenchymal tumor growth and in case of irregularly shaped tumors located in the central sections, laparoscopic intraoperative ultrasound of the kidney was performed, which made it possible to clarify the size, location and structure of the tumor, as well as its relationship to the CL.
Standard laparoscopic partial nephrectomy was performed at the stage of mastering LRP, as well as subsequently in complex cases, large tumors, intraparenchymal tumors and irregularly shaped tumors with a central location.
LRP using RFA without ischemia was performed when the tumor location was convenient for resection: extraorgan location in the lower or upper pole, along the anterior surface of the kidney and in the absence of proximity to the CL.
Standard LRP began with the formation of the intended resection line using scissors with monopolar coagulation. Renal ischemia was created by clamping the renal artery or the entire renal pedicle using a laparoscopic intracorporeal vascular clamp. In some cases, with a small superficial tumor, surgery was performed without clamping the renal vessels. Renal resection was performed using cold scissors and suction used to dissect the parenchyma and improve visualization of the resection margins. The resection margin was located at least 5 mm from the tumor edges. After a visual inspection of the bottom and edges of the wound, if necessary, coagulation of individual vessels was carried out with a monopolar ball electrode. When opening the mandibular joint, it was sutured with an atraumatic absorbable thread. A hemostatic mesh or Surgicel cotton wool was placed into the renal parenchyma defect, over which the renal parenchyma was sutured with atraumatic sutures (Vicryl 3.0). After completion of suturing of the renal parenchyma, the laparoscopic vascular clamp from the renal artery or pedicle was removed and the reliability of hemostasis was checked. The removed specimen (a section of the resected kidney with a tumor and adjacent perinephric tissue) was placed in a container and removed through a 2-3 cm mini-laparatomy incision at the location of the instrumental trocar. The perinephric tissue above the resection area was sutured with atraumatic sutures (Vicryl 3.0). The operation was completed by drainage of the retroperitoneal space.
In LRP using RFA, mobilization of the renal vessels was not performed. The renal pedicle was not clamped. Along the intended resection line, under visual and/or ultrasound control, a probe was inserted 5-7 mm from the edge of the tumor (Fig. 1).
Figure 1. RFA along the line of the proposed kidney resection. The ablation time for each point depended on the tissue resistance and averaged about 2 minutes. Kidney resection was performed using standard techniques without ischemia of the renal tissue.
In case of incomplete hemostasis along the resection line, additional ablation of the renal tissue was performed. After removal of the specimen and visual assessment of the resection margins, suturing of the renal parenchyma was not performed (Fig. 2).
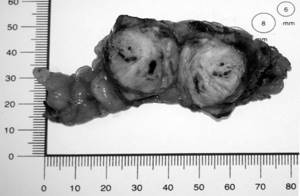
Figure 2. View of the removed specimen, a fragment of the kidney parenchyma with a tumor and adjacent perinephric tissue after LRP with RFA. The perinephric tissue above the resection area was sutured with atraumatic sutures (Vicryl 3.0). The operation was completed by drainage of the retroperitoneal space.
In the department of oncourology of the Moscow Research Institute of Oriology. P.A. Herzen in the period from 2003 to 2011, 122 LRP were performed. All surgical interventions were performed by one surgeon. Of the 122 operated patients, 74 (60.6%) were men and 48 (39.4%) were women. The average age of the patients was 53.8±11.6 years (16–79 years). A tumor of the right kidney was diagnosed in 62 (50.8%) patients, the left - in 59 (48.4%) and both kidneys - in 1 (0.8%) patient. LRP for a tumor of a single kidney was performed in 5 (4%) patients. The average tumor diameter, according to the preoperative examination, was 29.5 ± 11.4 mm (10–80 mm). The location of the tumor in 81 (66.4%) patients was predominantly extraorgan and in 41 (33.6%) with intraparenchymal growth. The distribution of tumors by segment was comparable: 42 (34.4%) tumors were localized in the middle segment of the kidney, 41 (33.6%) and 39 (32%) were localized in the upper and lower segments, respectively. The overall incidence of side effects was 14.75% (18 patients). After stratifying the patients into groups depending on the surgical procedure, they were compared. Statistica 8 program was used for statistical data processing.
results
51 (41.8%) patients underwent standard LRP and 71 (58.2%) patients underwent LRP using RFA. When comparing the groups according to the main preoperative indicators, it was revealed that the groups were comparable in average age, tumor size, tumor location and gender composition of the population (p>0.05) (Table 1).

The average age of patients was 53.1±11.6 years (27-75 years) in the standard LRP group and 54.4±11.6 years (16-79 years) in the LRP group with RFA. The average tumor size was 31.7 ± 11.5 mm (10–60 mm) in the standard LRP group and 28.1 ± 11.2 (11–80 mm) in the LRP with RFA group. In both groups, patients with an extraorgan tumor location predominated - 32 (62.7%) patients in the standard group and 49 (69%) in the LRP group using RFA, which was one of the selection criteria for the groups. However, no significant differences in tumor localization in different segments were identified.
When comparing the main postoperative indicators, there were no statistically significant differences in the time of surgical intervention, length of hospital day, frequency of complications and observation time (p>0.05) (Table 2).

Significant differences were detected only in the degree of blood loss and the size of the removed tumor according to the pathomorphological report. There was a trend toward a decrease in surgical intervention time in the group of patients who underwent LRP with RFA. The average operation time was 137.8±60.8 minutes (60-360 minutes) for the standard LRP group and 117.1±30.2 minutes (75-200 minutes) for LRP using RFA (p>0.05). The median blood loss when performing LRP using RFA was significantly lower and amounted to 100 ml, while blood loss in the group varied from 50 ml to 1100 ml, while in the group of standard LRP the median blood loss was 300 ml (50-2800 ml), p<0.001. Postoperative hospital stay ranged from 6 to 14 days in the standard LRP group and from 5 to 21 days in the group of patients who underwent LRP with RFA, while the median in both groups was comparable and amounted to 8 days (p>0.05).
In 104 (85.2%) cases, surgery was carried out without complications. 18 (14.8%) patients experienced intra- or postoperative complications. The groups were comparable in the incidence of intra- and postoperative complications. Complications were 11.8% (6 patients) in the standard LRP group and 16.9% (12 patients) in the LRP group using RFA (Table 3).
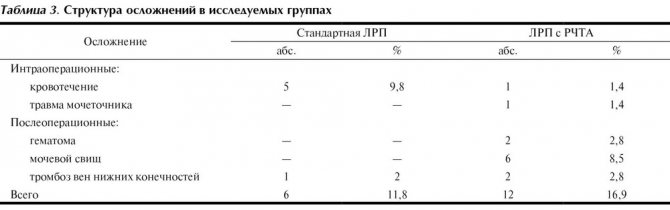
Complications mainly occurred at the stage of mastering the technique. In the group of patients with standard LRP, 4 (7.8%) patients underwent conversion due to bleeding; in the group of LRP with RFA, conversion was not performed, which was due to later mastery of the technique and increased experience in laparoscopic operations.
The main complications in patients who underwent LRP using RFA included the formation of a urinary fistula in 6 (8.5%) patients in the early postoperative period, hematoma in 2 (2.8%) patients, ureteral injury requiring stenting and suturing of the ureter atraumatic thread, in 1 (1.4%) and damage to the inferior vena cava, which led to greater blood loss (1100 ml) and required clipping of the defect, in 1 (1.4%) patient. Urinary fistulas were closed conservatively by performing ureteral stenting. One of the 2 patients with hematoma required drainage. The relatively high incidence of urinary fistulas in our observation is due to the fact that initially LRP with RFA was used in a large group of patients, including patients with tumors close to the CL. After reviewing the indications for the use of this technique, urinary fistulas were not observed in the latest sample of patients. When analyzing the relationship between the frequency of complications and the location of the kidney tumor (intraparenchymal or extraorgan), a weak statistically significant correlation was revealed: (correlation coefficient R = 0.25; Spearman method; p < 0.01). There was no significant correlation between the likelihood of complications and the localization of the tumor in a particular segment of the kidney (correlation coefficient R=0.15; Spearman method; p≥0.05). When assessing the possibility of the influence of tumor size on the likelihood of complications, no correlation was also found.
After receiving a routine histological report, statistically significant differences in the size of the removed kidney tumor were revealed. The average tumor size in the standard LRP group was 28.6±11.6 mm (10-70 mm), in the LRP group with RFA - 24.1±12.9 mm (5-85 mm). The larger tumor size in the population of patients who underwent standard LRP is due to the selection of patients and the need to perform surgical intervention under conditions of anoxia due to the larger tumor size, proximity of the CL and the greater likelihood of complications. In the standard LRP group, surgical intervention with anoxia of the renal parenchyma was performed in 25 patients, with the average anoxia time being 21.6±8.7 minutes (8-40 minutes). The observation time in both groups did not differ significantly, but was slightly longer in the standard LRP group, which is associated with the later development of the LRP technique with RFA. The average follow-up time in the standard LRP group was 49.3±40.2 months (1-102 months), and in the LRP group with RFA - 31.7±16.7 months (1-63 months).
According to pathomorphological examination, a clear cell variant of RCC was detected in 77 (63%) patients, a papillary variant in 16 (13%), a chromophobe variant in 13 (11%), a mixed variant of RCC in 3 (2%) and a mixed variant in 13 (11). %) - benign kidney tumors (Fig. 3).
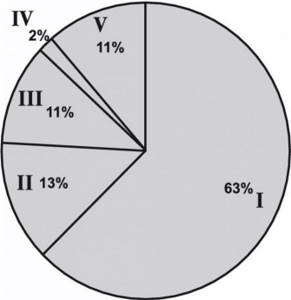
Figure 3. Variants of the histological structure of kidney tumors. I - clear cell; II - papillary; III - chromophobic; IV - mixed; V - benign formations. In our study, the following benign tumors were verified: oncocytoma - in 3 (2%) patients, angiomyolipoma - in 5 (4.5%), kidney cyst - in 5 (4.5%) patients. The distribution of patients by stage of the tumor process is presented in Fig. 4.
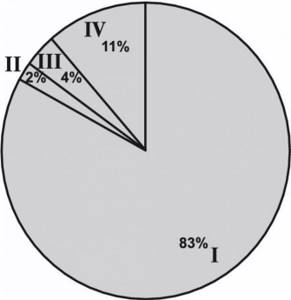
Figure 4. Distribution of patients by stage of the tumor process. I—pT1aN0M0; II - pT1bN0M0; III - pT3aN0M0; IV - benign formations. According to the pathomorphological report, stage pT1aN0M0 was verified in 101 (83%) patients, stage pT1bN0M0 - in 3 (2%) and stage pT3bN0M0 - in 5 (4%) patients. On day 7, the average postoperative creatinine level was 101.2 μM/L (70–156) in the standard LRP group and 121.9 μM/L (72–277) in the LRP with RFA group.
During observation, no local relapses or progression of the disease were detected; currently all patients are alive with good renal function. Neither warm nor cold ischemia was performed when performing LRP with RFA. No positive surgical margins were observed in any case.
Discussion
The widespread use of modern diagnostic methods (ultrasound, CT, MRI) in clinical practice now makes it possible to identify an increasing number of asymptomatic, small-sized kidney tumors.
Due to the migration of the stage towards localized RCC, the main treatment method for this group of patients has become organ-sparing operations, such as kidney resection in various options: standard, laparoscopic or robot-assisted. Oncological results when performing kidney resection do not differ from the results of radical nephrectomy, at the same time, organ-sparing operations are characterized by better functional results, especially in patients with initial impaired renal function, a single functioning kidney, or the presence of concomitant urological pathology. It is for this reason that organ-sparing operations for kidney tumors, including LRP, have recently become the most promising method of surgical treatment of patients with RCC with small (less than 4 cm, stage T1a) kidney tumors [1]. Despite the attractiveness of the concept of minimally invasive and minimally traumatic intervention for small kidney tumors, LRP in most clinics is not used as a standard method of surgical treatment of patients with RCC [1, 12]. Doubts about the effectiveness of LRP are due to technical difficulties in achieving reliable hemostasis during surgery and uncertainty about the radicalism of the intervention. Nevertheless, large urological oncology clinics have already accumulated sufficient experience in LRP, which allows one to evaluate the advantages and disadvantages of the method in comparison with open kidney resection [3, 8, 9]. The low morbidity of the laparoscopic approach during kidney resection, better cosmetic effect, reduction in hospitalization time and the number of analgesics used in the postoperative period are the undoubted advantages of this method compared to open surgery.
Ischemia of the renal parenchyma is not a prerequisite for surgical intervention, but it helps reduce blood loss and facilitates manipulation of the kidney, especially for tumors with intraparenchymal spread. B. Guillonneau et al. [10] note a statistically significant decrease in the average volume of blood loss in the group of patients who underwent clamping of the renal artery during LRP (270.3±281 ml), compared with patients who underwent resection without ischemia (708.3±569 ml). At the same time, the duration of the operation in the group with temporary cessation of renal blood flow also turned out to be less than in the group of patients without artery clamping - respectively 60-120 minutes (on average 121.5±37 minutes) in group 1 and 90-390 minutes (in average 179.1±86 min) in group 2, p=0.004. The duration of renal ischemia in this study was 15-47 minutes (average 27.3±7 minutes), which did not lead to a significant deterioration in renal function after surgery; the average serum creatinine level was 1.45±0.61 mg/Dl (128 ±54 µM/l) [10]. Other studies also confirm that renal artery clamping during renal LRP results in more effective hemostasis, reduces intraoperative blood loss, and facilitates renal manipulation. At the same time, J. Rassweiler et al. [13] consider it advisable to apply a clamp to the renal pedicle only for tumors larger than 2 cm or with significant intrarenal extension of the tumor. For small “exophytic” tumors, the authors performed laparoscopic organ-sparing surgery without creating renal ischemia.
The largest study comparing open partial nephrectomy (OPR) and LRP was performed by I. Gill et al. [4], who compared the results of treatment of 771 patients from the LRP group with the results of treatment of 1028 patients who underwent ORP. According to multivariate analysis, LRP was associated with shorter operative time, less blood loss, short hospitalization period (p<0.0001), but with long ischemic time and a high incidence of postoperative complications (p<0.0001) (Table 4).

In the majority of patients who underwent ARP, tumors were more than 4 cm in diameter and centrally located (p<0.0001). Renal function 3 months after surgery was comparable in both groups: LRP - 97.9% and ORP - 99.6% [4].
In another study, I. Gill et al. [5] from 1999 to 2008 in 800 patients who underwent LRP, the course of the postoperative period, the incidence of complications and renal function changed significantly due to different approaches to patient selection. The authors retrospectively divided 800 patients into 3 chronological groups: 1) September 1999 - December 2003, 2) January 2004 - December 2006, 3) January 2007 - November 2008 and assessed prospectively obtained information , including tumor characteristics, postoperative outcome and renal function. A comparison of periods showed that tumors in the later period were more often 4 cm in size or more and were centrally located, rarely less than 4 cm in diameter and peripherally located. At the same time, the warm ischemia time was shorter (31.9, 31.6 and 14.4 minutes, respectively, p<0.0001) and the incidence of postoperative and urological complications was significantly lower. Positive surgical margin rates were 1.0%, 1.0%, and 0.6%, respectively. Over the 9-year period of LRP, tumor characteristics and postoperative outcome varied significantly. Despite the increasing complexity of tumor removal in modern practice, such basic parameters as the postoperative period, ischemia time, complication rates and renal function have improved significantly. Currently, the authors perform LRP for tumors that were previously removed during ORP [5].
When comparing our results with literature data, a similar situation is noted, characterized by a decrease in the frequency of complications (11.8%), a decrease in the median blood loss (100 ml) and the average time of surgical intervention (117.1 min) due to the accumulation of experience and a new technique that allows achieve excellent hemostasis without ischemia of the renal tissue. Undoubtedly, standard LRP with clamping of the renal vessels is indicated for large kidney tumors with invasion into the renal joint and for central and intraparenchymal location of the tumor.
Thus, a comparative analysis of standard LRP and LRP using RFA showed significant advantages of the latter in the formation of intraoperative hemostasis using previous RFA when performing organ-saving operations on the kidney.
The main advantages of the technique include: no need for ischemia of the renal parenchyma, easier tumor removal, reduced blood loss and operation time, as well as additional radiofrequency treatment of the resection margin for ablative purposes.
Possible complications during and after surgery
- bleeding - usually blood loss during resection is about 500 ml or less, however, more serious blood losses occur that require the infusion of special solutions or blood transfusions;
- infection – to prevent the occurrence of infectious complications, a broad-spectrum antibacterial drug is administered before and after surgery, which reduces the risk of this complication to a minimum;
- damage to neighboring organs is an extremely rare complication; a several-fold increased view of the surgical field helps to avoid it;
- postoperative hernia - as well as organ damage are rare, due to the fact that the holes after laparoscopic operations are small;
- conversion (transition to open surgery) - occurs when it is impossible to perform laparoscopic intervention due to adhesions or bleeding.
What to expect after surgery?
After laparoscopic resection of the kidney, the patient is transferred to the intensive care unit (ICU) under the supervision of an anesthesiologist-resuscitator to monitor vital functions (blood pressure, heart rate, respiratory function, amount of urine excreted are monitored), in addition, discharge is assessed by drainage ( special tubes inserted into the wound cavity), temperature, urine color and general well-being of the patient.
Most often in the postoperative period there are complaints about:
- pain in the area of the postoperative wound - most patients experience minor pain, which often does not require drug pain relief, which differs from pain during open surgery, when analgesic therapy is required;
- nausea - most often a consequence of the administration of various drugs necessary for anesthesia;
- the presence of a urethral catheter - necessary to control the color and amount of urine; it is removed after a few days.
Causes of kidney cancer
Kidney oncology is the proliferation of a malignant neoplasm of an organ. The tumor may appear on one kidney or both. Metastases tend to be detected in other organs. Experts say that the occurrence of kidney cancer is associated with many provoking factors.
- Heredity – affects the genetic predisposition of the body to the development of the disease. If the parents suffered from cancer, then the children are more likely to develop malignant neoplasms.
- Often cancerous tumors appear due to exposure to radiation or frequent contact with harmful chemicals.
- Smoking promotes the penetration of a large number of carcinogens into the body. Most patients diagnosed with kidney cancer are addicted to this addiction.
- Obesity promotes the appearance of tumors in the kidney tissue at an early stage of the appearance of excess weight. Addiction to fatty foods increases the risk of cancer.
- Diabetes mellitus and hypertension can also become a provoking factor in the development of kidney cancer.
- Any harsh mechanical impact on the kidneys can provoke disease. Falls, injuries, and blows to the kidney area can cause cancer.
- Excessive drug use and uncontrolled use of drugs increases the risk of kidney cancer.
- Chronic diseases of viral etiology and problems associated with their treatment provoke the appearance of malignant tumors.
No one has yet established a clear cause for the occurrence of malignant neoplasms. There are many points of view on this issue. Given the undoubted success in the treatment of kidney cancer in Moscow, it is still too early to talk about a complete, 100% victory over cancer. Scientists will be able to defeat cancer only after the universal mechanisms of carcinogenesis and ways to prevent the development of malignant tumors are discovered.
What to do in the postoperative period?
On average, a patient stays in the hospital for about a week after laparoscopic partial nephrectomy. Drinking and eating are usually allowed the next day after surgery, and walking is allowed in the evening of the same day. After the operation, as before, a broad-spectrum antibacterial drug is administered.
During the postoperative period, you will be advised to:
- drink 1-2 liters of water per day;
- do not lift weights exceeding 5 kg;
- do not undergo heavy physical activity.
You should immediately consult a doctor if you have:
- blood appeared in the urine;
- body temperature increased;
- severe abdominal pain occurred.
After surgery, your doctor will schedule consultations for you to undergo examinations, blood and urine tests, ultrasound examinations, and computed tomography scans to evaluate the effectiveness of treatment. After 5 years, if there is no evidence of tumor progression, the patient is removed from the register.
Make an appointment at the urology clinic of the First Moscow State Medical University named after. Sechenov, you can see a urologist, oncologist, Doctor of Medical Sciences G. N. Akopyan by phone or through the interactive Appointment form on our website.
September 11, 2019
Hakobyan Gagik Nersesovich - urologist, oncologist, MD, doctor of the highest category, professor
All clinical observations...