Review on X-ray diagnosis of pneumothorax
OConnor A.R., Morgan W.E.
Spontaneous pneumothorax is relatively common [1]. The incidence of iatrogenic pneumothorax is difficult to estimate but is likely to be increasing due to the widespread use of mechanical ventilation (MV) and interventional procedures such as drainage and lung biopsy. In such cases, correct interpretation of the chest x-ray in a clinical setting is necessary and, if necessary, the use of more complex research methods. This article will discuss the role of x-ray in diagnosing pneumothorax before and after treatment, as well as the value of computed tomography and x-ray-guided drainage placement.
Diagnosis of pneumothorax before treatment
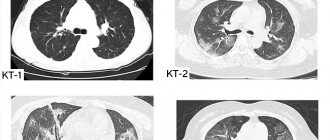
Pneumothorax is usually clearly visible on x-rays (Fig. 1). The visceral pleural line is visible without a peripheral pulmonary pattern. To diagnose doubtful cases, it is recommended to do an X-ray examination in the lateral projection or in the supine position [2]. On a standard lateral X-ray, the visceral pleural line may be seen in a retrosternal position or lying on the vertebrae, parallel to the chest [3]. Lateral or supine X-rays may be done in patients on a ventilator or in newborns. Although the assessment of respiratory function is controversial [4], many clinicians find it useful in identifying small pneumothoraxes when radiographic parameters are normal but the presence of pneumothorax cannot be ruled out. According to the recommendations of the British Thoracic Society [2], pneumothorax is divided into large (more than 2 cm) and small (less than 2 cm); the distance is calculated from the visceral pleura (edge of the lung) to the chest wall (wall). A small air edge around the lung actually translates into a fairly large reduction in lung volume; with a pneumothorax depth of 2 cm, it occupies almost 50% of the hemothorax [2]. Extensive pneumothorax is an objective indication for drainage [2].
In bedridden patients, air in the pleural space is usually easily visible at the base of the lung (Fig. 2), in the cardiophrenic recess and can lead to an increase in the costophrenic angle (a sign of deep grooves). Adhesion of inflamed pleura to the chest wall can limit pneumothorax by localizing part of the pleural space around the site of air leakage (Fig. 3). Drainage away from this area is not effective. If the surgeon introduces drainage into the area of pleural adhesion, this can lead to damage to the lung parenchyma and further release (leakage) of air (Fig. 4). For this reason, according to the authors, the approach to chamber (local) pneumothorax should be guided by fluoroscopy and, in some cases, computed tomography. Emphysematous bullae may also resemble chamber pneumothorax, particularly in the presence of chronic pulmonary disease. Sometimes the use of bright light helps to distinguish the internal pulmonary pattern in the bulla. If the clinical picture of the disease raises certain doubts, a computed tomography scan is necessary.
The chest radiograph should be carefully examined for the presence of underlying parenchymal pulmonary disease (Figure 5). The most common diseases predisposing to the development of pneumothorax are emphysema, pulmonary fibrosis of any etiology, cystic fibrosis, rapidly progressing pneumonia or pneumonia with the collapse of lung tissue, cystic lung diseases such as Langerhans cell histiocytosis and lymphangiomyomatosis. Identification of these diseases is very important because: firstly, parenchymal pulmonary disease is treatable; secondly, in contrast to primary spontaneous pneumothorax, patients diagnosed with secondary pneumothorax require careful hospital monitoring [2]. Finally, all but the smallest secondary pneumothorax (defined as apical or less than 1 cm in depth) requires treatment, even if symptoms are minimal [2].
Some known artifacts may resemble pneumothorax and should always be taken into account when interpreting an x-ray. The medial edge of the scapula may imitate the edge of the lung, but upon closer examination, a single combination of the edge of the scapula and the rest of the scapula can be traced (Fig. 5). Skin folds on the outside of the chest (Fig. 6) may resemble the visceral pleural line and, with the relative blurring of the pulmonary pattern in the upper sections, lead to incorrect diagnosis, especially in children. Skin folds are usually straight or minimally curved and do not run parallel to the chest wall like a true visceral pleural line. Clothes or sheets can produce a similar deceptive effect. In contrast to the loose visceral pleural line, the skin folds form a fairly dense line - pronounced on one side and blurred on the other. This last distinction may, however, be quite subjective. In some cases, it leaves a certain amount of doubt. In such a situation, a repeat X-ray examination should be performed, changing the position of the arm and removing excess clothing. Radiopaque lines often reflect the internal boundaries of the ribs, which can be mistaken for the visceral pleural line. They are often called associated opacities, although some authors use this term to refer to the density in the area of the first and second ribs. They are caused by protruding extrapleural fat or subcostal groove. The so-called accompanying opacification is characterized by a clear connection with the inner border of the accompanying rib, while the visceral pleural line deviates from the rib, forming a parallel line with the chest. Associated darkenings, located, as a rule, close to the adjacent rib, can sometimes protrude to some distance, leading to a certain confusion (Fig. 7). After pleurectomy for recurrent pneumothorax, a radiopaque line can be traced in the surgical field along the suture material or staples (Fig. 8). The latter may be misinterpreted as a new air leak, especially in comparison with x-rays taken the day before surgery or due to ignorance of the medical history and previous surgery.
Diagnosis of pneumothorax after treatment
After drainage, an x-ray examination is necessary, thanks to which you can monitor the resorption (resolution) of pneumothorax, determine the presence of complications and ensure the correct placement of the drainage. With a superficial dissection of tissue at the drainage site, subcutaneous or intramuscular introduction of drainage (which can be determined by palpation) will lead to extrapleural installation of drainage and ineffective treatment. This most often occurs when drainage is planned along the posterior surface of the chest, and the anterior x-ray appears satisfactory (Fig. 9). A lateral chest x-ray or computed tomography is necessary. The length of the drainage must also be correctly selected so that all side holes are located in the pleural space. Incorrectly selected drainage can lead to impaired drainage and the release of air into the subcutaneous tissue. The length of the tube with side holes can be determined using standard chest surgical drains by the distance of the radiopaque line (Fig. 10). Once the pneumothorax has resolved satisfactorily, the drainage catheter is removed and an additional chest X-ray is performed. There is usually a straight radiopaque line along the line of the preexisting catheter, known as the “drainage path” (Figure 11). It may be misinterpreted as a possible recurrence of pneumothorax, but its precise direct course and clear relationship with the location of the drain identified on x-ray before its removal usually helps to make a correct conclusion. Most likely, this is due to drainage imprints on the pleural tissue.
Once the drain is installed, it is attached to an underwater drain or vibrating valve [7]. The patient usually undergoes daily x-rays until the pneumothorax resolves. It is necessary to make sure that when conducting an x-ray examination, an unclosed drainage bottle is not placed above the level of the patient’s chest. This can lead to the accumulation of air and fluid in the pleural area and the appearance of hydropneumothorax. This should be taken into account if the patient’s condition suddenly worsens (especially in the absence of any clinical signs). This problem can be avoided if medical personnel pay great attention to the issue of positioning the drainage bottle.
Closing the drain with a clamp before x-ray examination is often done to detect minor leakage. The British Thoracic Society recommends that this method should not usually be used, but it can be done if the medical staff have the appropriate experience [8].
CT scan
The main purpose of computed tomography (CT) in this clinical setting is to differentiate between an emphysematous bulla and a pneumothorax, which can be quite challenging with standard radiographic examination. The high resolution of computed tomographs also makes it possible to detect parenchymal pulmonary disease (if present), which cannot be clearly verified by chest x-ray. CT always clearly tracks the route of insertion of an extrapleural or intrapulmonary catheter. Layered cross-sectional imaging is sometimes necessary when draining chamber pneumothorax located in hard-to-reach places.
Drainage under X-ray control
Chamber pneumothoraxes are best accessed using a puncture needle inserted under fluoroscopic guidance. The patient is usually placed on his back under the image intensifier, which makes the examination more comfortable for both the patient and the doctor. Small apical pneumothorax in patients with chronic lung diseases, especially in the presence of pleural adhesions, can be punctured through the axilla. With this approach, the patient is seated in a chair and the image intensifier is rotated around the patient to obtain a lateral view. Sometimes, in cases where it is impossible to make a lateral projection, it is necessary to perform a puncture in the second or even first intercostal space (Fig. 12). Small 8- to 10-gauge coil drains with a closure suture device are the most commonly used catheters in our department. They are suitable for cosmetic reasons and are easier to use than large 20 or 28 gauge drains when suctioning small amounts of air. In addition, along with large catheters, small catheters have proven their effectiveness in the treatment of pneumothorax [2]. However, pulling small sutureless catheters by the drainage bottle can cause rapid prolapse with prolapse of the side holes. When using such catheters, they must be well secured with adhesive tape. If the drainage is placed for a long time (more than 24 hours) or the patient cannot hold it himself, then the catheter must be firmly secured with suture material.
Abstract prepared by V.D. Sokolova
Based on an article by AR O'Connor, WE Morgan
"Radiological review of pneumothorax"
BMJ Vol. 330, 25 June 2005, pp. 1493–1497
Literature
1. Melton LJ, Hepper NG, Offord KP. Incidence of spontaneous pneumothorax in Olmsted County, Minnesota: 1950 to 1974. Am Rev Respir Dis 1979;120:1379–82.
2. Henry M, Arnold T, Harvey J. BTS guidelines for the management of spontaneous pneumothorax. Thorax 2003;58(Suppl 2):ii39–52.
3. Glazer H, Anderson DJ, Wilson BS, Molin PL, Sagel SS. Pneumothorax: appearances on lateral chest radiographs. Radiology 1989;173:707–11.
4. Seow A, Kazerooni EA, Pernicano PG, Neary M. Comparison of upright inspiratory and expiratory chest radiographs for detecting pneumothoraces. Am J Roentgenol 1996;166:313–6.
5. Kurihara Y, Yakushiji YK, Matsumoto J, Ishikawa T, Hirata K. The ribs: anatomic and radiological considerations. Radiographics 1999;19:105–19;151–2. [Quiz.]
6. Meholic A, Ketai L, Lofgren R. Fundamentals of chest radiology. Philadelphia: WB Saunders, 1996:29–31.
7. Laws D, Neville E, Duffy J. BTS guidelines for the insertion of a chest drain. Thorax 2003;58:53–9.
8. Baumann MH, Strange C, Heffner JE, Light R, Kirby TJ, Klein J, et al. Management of spontaneous pneumothorax. An American College of Chest Physicians Delphi consensus statement. Chest 2001;] 19:590–602
X-ray examination techniques
If pneumothorax is suspected, visual examination and x-rays are the first choice.
When examining the patient, the diagnosis is confirmed in the following cases:
- the person tries to be in a forced half-sitting position (in this position the pain is not so pronounced);
- the spaces between the ribs are widened;
- cold sweat is visualized on the skin, a blue tint to the skin;
- there is severe shortness of breath.
Among other symptoms identified during the initial diagnosis: low blood pressure, displacement of the borders of the heart to an intact organ, tympanic sound.
The final diagnosis is made after the conclusion of a radiologist.
What the attending physician would like to know
- Confirm the presence of air in the pleural cavity and identify the cause
- Determine the volume of pneumothorax.
Spontaneous pneumothorax in a 17-year-old boy. A plain chest x-ray shows an avascular space that surrounds the left lung like a mantle (mantle-like pneumothorax); its medial border is the contour of the pleura. The paramediastinal part of the lung causes the unusually sharp contour of the aortic arch.
Tension pneumothorax in a 27-year-old man. CT scan shows a massive mantle-shaped pneumothorax on the right with displacement of the midline structures to the left, flattening of the right dome of the diaphragm and its downward displacement.
Treatment
Pneumothorax due to traumatic injuries is treated by thoracic surgeons. The person is hospitalized in specialized departments. Oxygen therapy is given before chest x-rays are performed because oxygen accelerates pleural air reabsorption. Therapy for the pathology in question depends on the size, type, and clinical picture of pneumothorax.
Primary spontaneous pneumothorax up to 20% in size without respiratory symptoms sometimes does not require treatment unless it progresses, which should be monitored radiographically. Significant or symptomatic primary spontaneous pneumothorax should be evacuated by chest drainage.
Drainage is performed by inserting a small-diameter intravenous needle or pigtail-type catheter into the second intercostal space along the midclavicular line. The catheter must be connected to a 3-way adapter and a syringe. The air is collected in a syringe and removed. The action must be repeated until the air is straightened or until four liters of air have been “pumped out”. When the lung expands, the catheter is removed, but in some cases it is left after the one-way Heimlich valve is attached, so the patient can move around while in the hospital.
If the lung does not expand, the pleural cavity is drained. For primary spontaneous pneumothorax, a chest tube attached to a container of water is sometimes first placed. Patients need to convey the message that they need to stop smoking, because this bad habit is a dangerous risk factor for pneumothorax.
For secondary and traumatic pneumothorax, in most cases it is necessary to drain the pleural cavity, but often cases are treated on an outpatient basis. For iatrogenic pneumothorax with severe symptoms, aspiration is most often done. Tension pneumothorax is an emergency. Treatment begins immediately: by inserting needles with a diameter of 14 or 16 gauge into the second intercostal space along the midclavicular line, which is then connected to the catheter. There is a sound of air being released under pressure. This confirms the diagnosis is correct. Emergency decompression is completed by inserting a thoracostomy tube, after which the catheter must be removed.