Magnetic resonance imaging (MRI) of the brain and spinal cord is the main diagnostic method used to confirm the clinical diagnosis of multiple sclerosis (MS). To confirm the diagnosis of MS, it is necessary to determine two main key characteristics: the dissemination of the pathological process in space and time. They formed the basis of the MRI criteria for MS, which appeared in the late 80s of the last century and were periodically revised in connection with the emergence of new knowledge and the results of clinical studies. One of the significant revisions of the McDonald MRI criteria occurred in 2005. These criteria existed until 2010, when, in order to simplify and speed up diagnosis, the 2010 McDonald MRI criteria were recommended for widespread use.
Application of the 2010 MacDonald MRI criteria.
Over the past 30 years, the neurological community has adopted various diagnostic criteria, which have been modified each time after the emergence of new clinical data [1–4]. As is known, treatment of any disease is most effective at its earliest stage, and therefore early, quick and accurate diagnosis of R.S. is especially important. The 2010 revision of the diagnostic criteria for MS is based on identifying lesions of the central nervous system (CNS) that demonstrate dissemination of the pathological process in space and time. In addition, diagnostic criteria require the exclusion of other alternative diagnoses [5, 6]. Thus, from a formal point of view, the diagnosis of MS can be made only on the basis of clinical manifestations, but an MRI study is necessary to confirm the key characteristics described above and exclude other pathologies of the central nervous system.
In 2010, the International Group MAGNIMS (Magnetic Resonance Imaging in MS) presented a revision of the McDonald criteria (Table 1). This option made it possible to increase the sensitivity of MRI criteria. However, the specificity of the method has decreased slightly compared to earlier revisions from 2001 and 2005. [3, 7]. In the 2010 MacDonald MRI criteria, when assessing the dissemination of a pathological process in space, the emphasis is not on the number of lesions, but on their typical location, which facilitates the interpretation of MRI. They also do not require a mandatory time interval between the clinical attack and the initial MRI examination, which facilitates an earlier start of patient monitoring. In addition, the concomitant presence of contrast agent (CM)-accumulating and non-accumulating lesions is taken into account as evidence of dissemination of the pathological process over time in some patients who underwent a single MRI study at any time after the onset of symptoms of the disease.
Table 1. McDonald's 2010 MRI criteria.
Previously, there was no evidence that the neuroimaging characteristics of patients with relapsing-remitting MS (RRMS) differ significantly from those of patients with primary progressive MS (PPMS) [8]. In the modified McDonald criteria of 2010, MRI criteria were first proposed separately for both the diagnosis of PPMS and RRMS (see Table 1) [3, 9]. According to these criteria, the dissemination of the pathological process in space in PPMS is determined by two of the following three criteria: the presence of 1 lesion or more in the brain in T2 modes, in at least one of the three localizations typical for MS (periventricular, juxtacortical, subtentorial); the presence of 2 or more foci in T2 modes in the spinal cord; positive cerebrospinal fluid (CSF) test for oligoclonal IgG antibodies.
Despite their advantages, MacDonald's 2010 MRI criteria have attracted criticism. It has been suggested that they may compromise diagnostic specificity, leading to overdiagnosis of RS. This risk is especially great when MRI findings are analyzed without consideration of clinical and laboratory information or are interpreted by radiologists and clinicians who do not have the necessary experience in assessing brain and spinal cord lesions. Diagnostic difficulties are also caused by the lack of “neurological” skills of the MRI specialist, who, according to the 2010 MRI criteria, needs to differentiate between symptomatic and asymptomatic lesions. It should be noted that CSF analysis is not required to confirm the diagnosis of RRMS according to the 2010 McDonald criteria, although relevant data can undoubtedly help some patients [10].
It should be recognized that the 2010 McDonald MRI criteria have significantly improved the process of diagnosing RRMS, but they have a number of limitations in PPMS [3]. In these cases, MRI of both the brain and spinal cord, despite the improvement of modern equipment, may not reveal any features or damage [11]. Thus, diagnosing PPMS can be significantly difficult.
As noted above, the 2010 revision of the MacDonald MRI criteria was based in part on the results of the MAGNIMS group studying Caucasian patients [9, 12]. There is an opinion that they can be used to diagnose MS in people of Asian, Hispanic origin, as well as pediatric patients [3]. Currently, data have already been published regarding the specificity of the McDonald criteria in patients of this age group. One such study retrospectively compared the 2005 and 2010 McDonald MRI criteria. in a group of children with acute demyelination, who were then followed prospectively over the next 24 months [13]. The researchers found high sensitivity and specificity of the 2010 McDonald MRI criteria in children over 11 years of age who had clinical symptoms not associated with acute disseminated encephalomyelitis (ADEM). The results and conclusions of another multicenter retrospective study [14] also confirmed the high diagnostic value of the 2010 McDonald MRI criteria in children.
After identifying Devic's neuromyelitis optica and neuromyelitis optica-associated syndromes, the accuracy of the 2010 McDonald MRI criteria in patients with clinically isolated syndrome (CIS) became comparable between Caucasian and Asian patients [15].
Application of MRI criteria for MS 2021
MRI criteria were first included in the diagnostic algorithm for patients with CIS in whom MS was suspected in 2001, and have since undergone several revisions. Since their update in 2010, not only new data have appeared on the use of MRI in assessing the dissemination of the process in space and time, but MRI equipment has also improved in the form of wider introduction into practice of high-field tomographs (1.5 and 3 Tesla) and new pulsed sequences. All this was the reason for the latest revision of the MRI criteria for MS by the MAGNIMS group in 2021.
These changes are reflected in table. 2, from which it is clear that they touched upon the criteria for dissemination of the pathological process in space.

Table 2. MAGNIMS 2021 MRI Criteria Note: For convenience, all changes in the table. 2 regarding the latest revision from 2010 are underlined.
Given that the presence of a single periventricular lesion is not sufficiently specific for a demyelinating inflammatory process and that lesions can be detected in healthy people and patients with other neurological diseases [16], changes were made to the number of periventricular lesions compared to the McDonald 2001 MRI criteria. and 2005 [12]. The importance of the number of lesions has been confirmed in a number of studies when analyzing a large cohort of 652 patients with CIS, which showed that in patients who do not meet the criteria for dissemination of the pathological process in space for MS, but have three periventricular lesions in combination with age and the presence of oligoclonal antibodies, there is a high risk of developing MS [17]. In another study [18], in patients with CIS involving the spinal cord, under the age of 40 years, with three or more periventricular lesions and intrathecal immunoglobulin synthesis, the development of MS was observed with an accuracy of 78%. In a multicenter study of 468 patients with CIS, the presence of 3 perivericular lesions had a significant prognostic value for transformation to MS over a 3-year follow-up period [19]. In a study comparing patients with MS and primary and secondary CNS vasculitis, the presence of three or more periventricular lesions was the only feature in the MRI criteria that differentiated MS from systemic lupus erythematosus or Sjögren's syndrome [20].
The literature also draws attention to the need to include the optic nerve as an additional area of the central nervous system typical of MS, which may be involved in the pathological process. Imaging features of clinically asymptomatic inflammation of the optic nerve (foci in it on MRI or thinning of the layer of retinal nerve fibers) confirm dissemination in space and, in patients without visual impairment at the moment, dissemination in time [8].
The results of histological studies have shown that in MS there is extensive involvement of gray matter in the pathological process [21]. In accordance with the localization of lesions in the cerebral cortex, the following subtypes of cortical lesions are distinguished: subpial, purely intracortical, and lesions located at the border of the cortex and subcortical white matter. Visualization of cortical lesions is quite challenging, especially when using standard MRI protocols. To improve the sensitivity of cortical lesion imaging, various special pulse sequences have been proposed, including DIR, PSIR, MPRAGE [22]. However, many cortical lesions remain invisible on MRI, even on tomographs with magnetic induction values of 1.5 and 3 Tesla [23]. In this regard, changes in MRI criteria were expressed not in the identification of a new, cortical, localization, but in the expansion of the term “juxtacortical localization,” which now sounds like “juxtacortical/cortical localization.” Evaluation of cortical lesions can also help in the differential diagnosis of MS from other diseases that mimic MS (for example, cortical lesions have never been detected in migraine and neuromyelitis optica, and are also very rare in healthy patients) [21, 22].
The 2010 MacDonald diagnostic criteria for assessing the dissemination of the pathological process in space did not take into account lesions localized in the brain stem and spinal cord. In the latest revision of the MRI criteria for MS, this limitation was removed and any lesions now have equal diagnostic value. To confirm spatial dissemination of the pathological process, imaging of the entire spinal cord is recommended (especially in patients who do not meet the criteria for spatial dissemination on MRI of the brain).
The division of the types of MS into RRMS and PPMS, as is known, is purely clinical. However, various biomarkers are being sought that could further distinguish between these clinical forms. In 2012, data were published on the sensitivity of using criteria for spinal cord injury and examining CSF for the presence of oligoclonal antibodies [24]. As a result of this study, the criterion for dissemination of the pathological process in space for the spinal cord in the MRI criteria for MS in the 2021 revision was changed from 2 lesions or more to 1 lesion or more (it does not matter whether these lesions are clinically significant or not) , which significantly simplified this criterion and increased its sensitivity (see Table 2). Nevertheless, a more detailed development of the criterion for the specificity of dissemination of the pathological process is required.
MRI criteria used to confirm the dissemination of the process in space and time in MS can also be used in radiologically isolated syndrome (RIS) [25].
Today, it is believed that MRI criteria for MS can be used not only in Caucasians, but also in patients from Asia and Latin America [14, 26]. But, unfortunately, there are no studies yet that would analyze the use of MRI criteria for MS in patients of African and Middle Eastern origin. In children over 11 years of age, in whom ADEM has been excluded, MRI criteria for dissemination of the process in space and time can be used, as in adults, but in children under 11 years of age, even in cases where ADEM has been excluded, their use should be taken more carefully [13] .
Histological and MRI data have shown that diffuse (and irreversible) damage to brain matter begins in the earliest stages of MS. Early detection of these changes can help identify patients at increased risk of developing severe disability. Standard MRI techniques, such as T2 modes and T1 modes after injection of CI, have high sensitivity in visualizing lesions in the white matter of the brain. However, they are not specific enough to determine the nature of changes within the lesion, and even more so, they do not have the necessary sensitivity to detect diffuse changes in the externally unchanged gray and white matter of the brain and spinal cord. Currently, the main task of researchers is to study and implement advanced MRI techniques aimed at identifying these changes.
One of these advanced MRI techniques is proton MR spectroscopy, which was performed in patients with CIS to determine the nature of changes in the brain substance in addition to the foci of demyelination visualized in T2 modes. In patients with CIS, a significant decrease in N-acetylaspartate (a marker of neuronal damage) as well as an increase in myoinositol (a marker of glial cell damage) was found in intact white matter (NWM). It is important to note that the level of these changes was higher in those patients who were subsequently clinically diagnosed with MS [27]. Diffusion tensor MRI and magnetization transfer imaging also revealed differences in the VNWV when comparing patients with CIS and controls. This suggests that these techniques may be of great importance in predicting cognitive impairment and disability in such patients [28, 29].
Although these advanced MRI techniques can provide information for assessing the risk of developing MS, their sensitivity and specificity for diagnosis and differential diagnosis in individual patients remain to be studied. They can be used to differentiate MS from other demyelinating diseases. Thus, it has been shown that in ADEM, Devic's neuromyelitis optica and Leber's optic neuropathy, less diffuse changes in the brain substance are observed than in typical MS [30, 31]. However, prospective studies are required to evaluate the advantages of new MRI techniques over standard MRI in the diagnosis and prognosis of MS in order to quickly make decisions regarding patient treatment.
CV-accumulating lesions represent new, currently active lesions, while non-CV-accumulating lesions visualized on T2 are older lesions of demyelination. In this regard, the criteria for dissemination of the pathological process over time must be met provided that lesions with different states of the blood-brain barrier are detected, regardless of whether they are associated with real clinical symptoms or not [32].
“Black holes” - hypointense foci that do not accumulate CV in T1 mode are areas in the brain substance with pronounced demyelination and axonal death, and are most often found in patients with a long history of the disease and progressive types of the disease. Such changes in T1 mode must also be taken into account in MRI criteria. Thus, the presence of “black holes” on MRI in patients with CIS may indicate a not entirely favorable course of the disease. However, it should be noted that they do not have prognostic value in the transformation of CIS into MS [33]. The presence of chronic “black holes” is not used as a potential alternative criterion for dissemination over time in adults, but is most justified in children in differentiating MS from a monophasic disease (for example, ADEM).
Recognizing the unconditional value of the MRI method in diagnosing MS, doctors should still draw the attention to the fact that some changes on MRI are not specific to this disease. In this regard, it should be noted that the MacDonald MRI criteria of recent revisions have become less strict and this, unfortunately, can sometimes lead to overdiagnosis of R.S. Therefore, issues of differential diagnosis are so relevant at present in the field of MRI application.
Potential targets for differential diagnosis are the periventricular localization of demyelination foci and the presumed increase in iron deposition in them [34]. These signs are especially visible on MRI with high magnetic flux density (≥3 Tesla) [35]. The SWI (susceptibility-weighted imaging) pulse sequence, first described in 2004 [36], has shown high sensitivity in identifying small cerebral veins and detecting iron-containing areas with paramagnetic properties. The use of SWI provides an additional opportunity for differential diagnosis, especially when SWI is performed and evaluated in conjunction with the FLAIR mode and is called FLAIR* (see figure) [37].
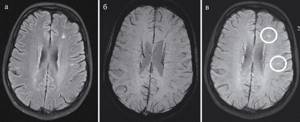
MRI of the brain of a patient with MS in FLAIR (left), SWI (center) and their combination - FLAIR* (right). Perivenular localization of demyelination foci is observed in FLAIR* (venocentric pattern).
Recent experience with the use of SWI on MRI with a magnetic induction value of 3-7 Tesla has shown that most chronic and some acute lesions of demyelination in MS may have areas of reduced MR signal intensity. These hypointense changes likely represent free radicals or iron deposits, although loss of myelin may also contribute to changes in MR signal intensity on SWI [38].
A significant portion (more than 40%) of MS lesions have a small vein in the central sections [39]. In a recent study using 3D T1 mode after the introduction of CV on an MRI scanner with a magnetic induction value of 3 T, the so-called venocentric pattern was observed in the majority of MS lesions and amounted to 95% [40]. Venocentric localization of lesions and the presence of hypointense areas in them are specific markers and should be used in the differential diagnosis of patients with CIS or MS with other neurological diseases [41]. In this regard, further research should be aimed at studying the above-described properties of SWI in order to increase the specificity of MRI in the diagnosis of MS.
Another direction in the differential diagnosis of MS is the identification of changes in cortical localization that are directly related to cognitive impairment. Cortical lesions are found in sufficient numbers in patients with MS and are easily visualized in the “double inversion recovery” (DIR) pulse sequence, in which the intensity of the MR signal from the white matter and CSF is suppressed [34]. Thus, DIR improves the sensitivity of MRI for detecting cortical lesions in
vivo
[42], but does not yet allow differentiating between types of cortical lesions [22]. More sensitive in this sense are the PSIR (phase-sensitive inversion recovery) and MPRAGE (high-resolution 3D magnetization-prepared rapid acquisition with gradient echo) sequences, performed on devices with high magnetic induction values. Thus, when these sequences are used together, at least one cortical lesion is detected in 36% of patients with CIS, which correlates with a high risk of transformation into clinically significant MS [43].
Repeated MRI studies of the brain should be performed in patients who have clinical and radiological data suggestive of MS, but do not yet fully meet the MRI criteria for MS. The time interval between the initial and subsequent MRI scans is still a matter of debate, but it is believed that the optimal interval should be 3-6 months. This assumption is based on the fact that the majority (80%) of patients with CIS who have at least three lesions in the white matter on the initial MRI study develop new lesions in T2 modes over the next 3 months [44]. If there are no new white matter lesions on follow-up MRI, a third scan can be performed after 6 to 12 months. These time intervals may also be used in patients with RIS. New active lesions that appear in patients with RIS on subsequent MRI studies significantly increase the risk of developing MS in these patients [45], although an accurate diagnosis of MS cannot be established in the absence of corresponding clinical symptoms.
The primary goal of repeat brain MRI examinations is to identify active lesions (ie, new or enlarged T2 lesions with or without T1 CV accumulation). It is important that repeat MRI scans are performed using the same equipment and the same standardized MRI protocol as the initial scan [46]. Adequate repositioning is also necessary for accurate assessment of compared sequential MR images and, conversely, improper repositioning can lead to artifacts that can mimic changes in MS.
Thus, to make a clinical diagnosis of MS, several components are important in MRI diagnostics: conducting an MRI examination by qualified specialists, taking into account the use of a standardized MRI protocol, including the introduction of CV to identify active lesions and differential diagnosis with other diseases, as well as interpretation of scan results a radiologist with sufficient experience in analyzing such images. Of course, even in the presence of multifocal brain damage with an MRI pattern characteristic of MS, it is necessary to compare MRI data with neurological symptoms and exclude other alternative diseases.
The authors declare no conflict of interest.
Disease Imaging
Because the diagnostic test is done by influencing hydrogen contained in the body, doctors know what multiple sclerosis looks like on an MRI. This is possible because human nerve cells are protected by a layer of myelin: a fatty structure that repels water. A disease such as multiple sclerosis destroys fat, so unprotected tissues are susceptible to the accumulation of excess moisture. It is water that is displayed in the image after scanning and looks like a shadow or, conversely, an illuminated area (the type of scanning is taken into account).
Diagnostic features
Previously, multiple sclerosis was diagnosed only clinically, based on symptoms. Today, the initial diagnosis requires confirmation. If the doctor makes a preliminary determination, a referral is given to find out what the magnetic resonance imaging will show.