What are the consequences of the state of hemoconcentration?
Why is hemoconcentration (blood thickening) dangerous during pregnancy? Both the mother and her unborn baby can suffer from this pathology. The doctor immediately puts this situation under control, since the following consequences may occur:
- Varicose veins,
- The appearance of blood clots
- Stroke,
- Heart attack.
For a child, this situation is even more dangerous. He faces:
- Oxygen starvation,
- Pathological development,
- Fading pregnancy
- Risk of miscarriage,
- Premature birth.
Risks for mother and baby
The main danger lies in the formation of blood clots.
Often, after childbirth, a new mother may develop varicose veins. Blockage of blood vessels can negatively affect both the fetus, which will experience oxygen deficiency, and the expectant mother herself. Because of this, the possibility of miscarriage or premature birth, heart attack or stroke may increase significantly. In the early stages, excessively thick blood can lead to a frozen pregnancy. Therefore, it is so important to visit a doctor on time and fully follow his recommendations. If you have any doubts about the prescribed treatment, immediately consult with several doctors, so you can eliminate the risk of medical error or negligence.
Is thick blood during pregnancy dangerous for the woman and fetus?
It is based on the results of a coagulogram that a specialist will be able to determine the degree of danger of blood thickening during pregnancy. In some cases, with minor changes in indicators, the doctor does not attach serious importance to blood density and gives the woman general recommendations regarding diet and fluid intake aimed at eliminating this symptom. In such situations, you should not worry, because such blood thickening does not pose a threat to either the expectant mother or the fetus, and after childbirth, the coagulogram indicators stabilize on their own.
Sometimes the cause of blood thickening during pregnancy is taking iron-containing drugs, which are prescribed when hemoglobin levels decrease. Such a symptom should also not cause concern in a woman, because after eliminating anemia and discontinuing these medications, the blood condition will stabilize.
For more serious changes in coagulogram parameters, the doctor may recommend that the pregnant woman undergo a course of blood thinning therapy. In such situations, a woman should also not worry, but simply follow all the doctor’s orders. The danger of such blood thickening lies in the increased risk of blood clots and obstructed blood flow through the vessels, but this situation can be corrected.
The slow flow of viscous blood through the vessels and a more intense load on the heart causes insufficient supply of oxygen and nutrients to all tissues and organs. This leads to the following symptoms in a pregnant woman:
- constant lethargy;
- memory impairment;
- drowsiness;
- dry mouth;
- heaviness in the legs;
- coldness of the extremities.
With a sedentary lifestyle and lack of treatment, an increased tendency to blood clots can lead to the development of the following complications in the expectant mother:
- thrombosis;
- thrombophlebitis;
- TELA;
- varicose veins;
- diseases of the cardiovascular system (heart attack, hypertension, stroke, atherosclerosis).
Significantly increased blood density also negatively affects the condition of the unborn baby. As a result of increased thrombus formation and slow blood flow, the following disorders may occur on the part of the fetus:
- miscarriage or premature birth;
- frozen pregnancy;
- hypoxia;
- developmental delay.
It is in connection with the above possible complications of thick blood that women planning a pregnancy should refuse to conceive until the course of treatment for this condition is completed. In some cases, this disorder in the blood coagulation system can be life-threatening for the expectant mother and baby, and not all medications can be taken by a woman while carrying a child. Therefore, it is better to get rid of this symptom before pregnancy.
- Thick blood what to do: how to thin thick blood
When planning a conception, the doctor will definitely prescribe a coagulogram to rule out disorders in the blood coagulation system. It is especially important to conduct such a study in certain risk groups:
- the woman has a history of miscarriages or missed pregnancies;
- the woman or her relatives have varicose veins;
- close relatives of the woman had thrombosis, heart attacks or strokes;
- a woman professionally engages in sports that involve intense physical activity.
Coagulogram - what kind of analysis is this?
The functioning of the circulatory system is perhaps the most important indicator of health. During pregnancy, she requires increased attention. A coagulogram reveals disorders of hemostasis - the system that is responsible in our body for the normal liquid state of blood and for blood clotting to stop bleeding. If hemostasis indicators are reduced, a person will experience large blood losses even due to a minor cut. Too high (hypercoagulation) leads to thrombosis, heart attacks and strokes.
According to doctors, the danger of both anomalies is that they may not reveal themselves in any way in everyday life. But during surgery or childbirth there is a risk of developing a critical situation.
The hemostatic system undergoes some changes during pregnancy. Therefore, the coagulogram before and during pregnancy is different. Nature provides for such a natural restructuring. In the process of bearing a child, a third circle of blood circulation arises in a woman’s body, which is called the uteroplacental. Preparations are underway to increase the amount of circulating blood. This is important also because of the inevitable blood loss during childbirth.
Causes
While carrying a baby, the expectant mother is faced with a huge number of tests. A general blood test and hemocoagulogram are mandatory tests that are included in the recommended list of laboratory tests performed during pregnancy.
“Thick” blood is a clinical concept that is defined when the amount of formed elements in the blood is significantly increased. Typically, various thrombus formation disorders lead to the development of this situation during pregnancy. These pathologies can appear both in the early and late stages of pregnancy.
It should be noted that normal blood is quite liquid. This physiological feature is necessary for its transport and nutritional functions to be fully realized.
There are quite a lot of different nutrients dissolved in the blood, as well as oxygen. All these elements are needed by the fetus for its active growth and development.

The development of pathological disorders associated with the formation of thick blood is caused by the following causes:
- Individual characteristics of the expectant mother. If a woman had any hematological disorders before pregnancy, then during pregnancy they will progress significantly. This situation usually occurs in families where several members have various diseases of the cardiovascular system. A history of heart attack or stroke in close relatives of a pregnant woman is also a predisposing factor to increased thrombus formation.
- Violation of the drinking regime. Insufficient intake of water into the expectant mother's body can lead to her blood becoming thicker. This violation occurs quite often if a woman suffers from toxicosis. Frequent vomiting contributes to dehydration, which leads to severe blood thickening.
- Insufficient supply of essential vitamins and microelements. Vitamin balance is very important during all periods of pregnancy. Carrying a baby is a very energy-intensive time. To carry out all biological reactions, enzymes are needed, which cannot be formed in the mother's body without certain vitamins and microelements.
- Frequent consumption of sweets and other “fast” carbohydrates . A large amount of sugar entering the blood leads to a significant change in its viscosity. If the expectant mother eats a lot of sweets and candies throughout her pregnancy, this can not only contribute to increased blood clot formation, but even lead to the development of signs of diabetes mellitus.
- Oversaturation of the body with iron-containing drugs . These drugs are usually prescribed to pregnant women who have had a decrease in hemoglobin during pregnancy. Excessive intake of iron-containing drugs can lead to an increase in platelets in peripheral blood.
- Impaired functioning of the spleen . This organ is necessary for the body to maintain optimal concentrations of blood cells. Hypersplenism is a pathological condition that is characterized by significant disturbances in the functioning of the spleen.
This pathology, which occurs during pregnancy, also contributes to the progression of thrombosis.
It is important to note that there are a number of specific pathologies that occur mainly only during pregnancy. antiphospholipid syndrome can lead to an increase in blood viscosity and disruption of its fluidity Doctors note that the incidence of this pathology is only growing every year.
Severe blood loss or traumatic shock resulting from some type of injury may also cause baseline blood counts to change. These pathologies can also appear if a pregnant woman shows signs of internal bleeding. This condition is already extremely unfavorable and requires urgent medical attention.
- Finger joints hurt during pregnancy: causes of pain in the hands
Calculate gestational age
Physiology of the hemostatic system and its features in uncomplicated pregnancy
The hemostasis system, or the system for regulating the state of blood aggregation (RAS), O.K. Gavrilov (1) defined it as “a complex of selectively involved components in which the interaction and relationships, despite their opposite nature, take the form of interaction in obtaining a focused beneficial result - the hemostatic potential of the blood, ensuring the preservation of the fluid state or blood coagulation.”
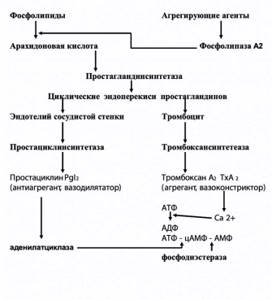
Figure 1. Vascular-platelet link of hemostasis
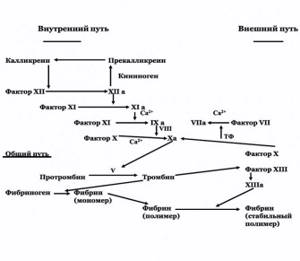
Figure 2. Procoagulant part of hemostasis. Extrinsic blood clotting pathway
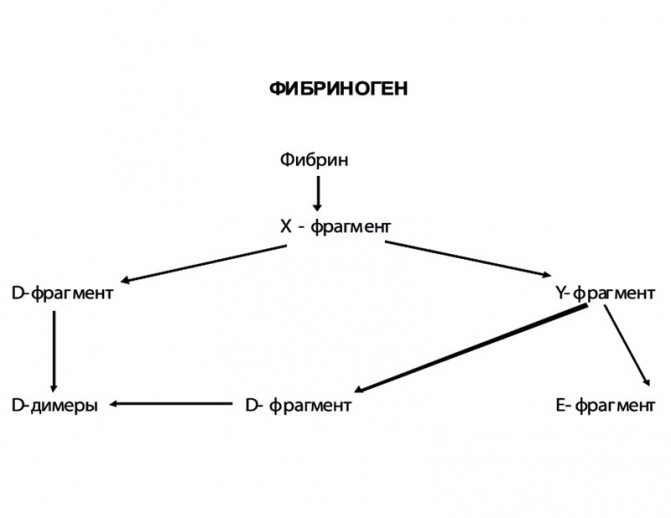
Figure 3. Fibrinolysis pathways
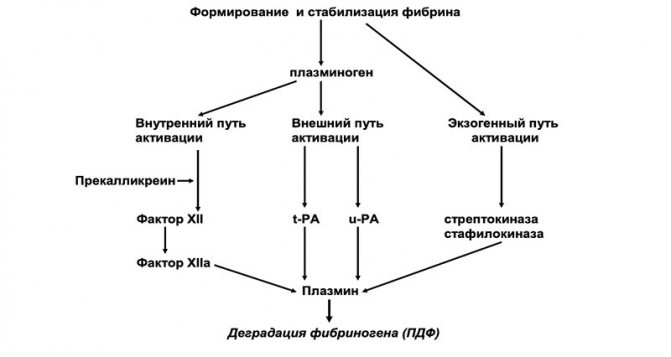
Figure 4. Formation of PDF
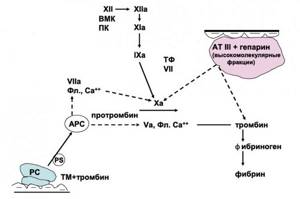
Figure 5. Protein C system

The RAS system is mosaic, that is, the hemostatic potential in different parts of the blood flow, in different organs, is not the same. This is the normal state of the RAS functional system. One of the main features of the RASK system is the interaction of coagulation proteins with membrane surfaces and with metal ions. The role of phospholipids, primarily phosphatidylserine, is important.
The second feature is the speed of the system using positive and negative feedback mechanisms. High response rates and speed of reactions are achieved due to the basal level of circulating coagulation enzymes.
The third feature is the limited response in terms of localization and duration of exposure, which is of great importance.
The fourth feature is the high integration of the RAS system with other protective blood systems, including the complement system and the cytokine system (interleukin 1-β, tumor necrosis factor-α) (2).
Schematically, the hemostasis system is represented by the following components:
- vascular complex (primarily endothelium) and platelet link;
- a link in procoagulants;
- fibrinolytic link;
- part of blood clotting inhibitors.
The most important element of the vascular wall is the endothelium. The antithrombotic activity of the endothelium is due to the synthesis of prostacyclin (PGI2), a powerful inhibitor of platelet aggregation, nitric oxide (NO), tissue plasminogen activator (t-PA), antithrombin III (AT III), extrinsic coagulation pathway inhibitor (TFPI), and thrombomodulin.
According to Z.S. Barkagan (3), in addition to these functions of the endothelium, there are a number of features: inability to contact activation of the blood coagulation system; creation of an anticoagulant potential at the blood/tissue interface by fixation of the heparin-antithrombin III complex on the endothelium, the ability to remove activated coagulation factors from the bloodstream.
The participation of platelets in hemostasis is determined by their ability to adhere to the site of endothelial damage, the process of their aggregation and formation of a primary platelet plug, their ability to maintain vascular spasm through the secretion of vasoactive substances - adrenaline, norepinephrine, serotonin, ADP, etc., as well as to form, accumulate and secrete substances that stimulate adhesion and aggregation.
Adhesion (sticking) of platelets to the site of damage to the vascular wall is a reversible process. Platelet aggregation occurs simultaneously with adhesion.
To a large extent, the mechanism of platelet aggregation became clear after the discovery of prostaglandins in platelets and the vascular wall. It turned out that various aggregating agents activate phospholipase A2, which causes the cleavage of phospholipids from arachidonic acid, a powerful aggregating substance (Figure 1). Under the influence of prostaglandin synthetase, cyclic endoperoxides of prostaglandins I2 and H2 are formed, stimulating the contraction of platelet microfibrils and having an aggregating effect. Thromboxane A2 is synthesized in platelets under the influence of thromboxane synthetase. The latter promotes the transport of Ca2+ in the platelet, which leads to the formation of ADP, the main endogenous stimulator of aggregation. The level of Ca2+, as well as the level of cAMP, a universal biological transporter, is regulated by adenylate cyclase, which catalyzes the ATP-cAMP reaction.
In the endothelium, under the influence of prostaglandin synthetase, arachidonic acid is converted into prostaglandin endoperoxides (similar to these processes in platelets). Further, under the influence of prostacyclin synthetase, prostacyclin (prostaglandin I2) is formed, which has a powerful disaggregating effect and, in addition, activates adenylate cyclase (4).
Thus, the so-called thromboxane-prostacyclin balance is one of the main regulators of the tone of the vascular wall and platelet aggregation.
Based on their functional and structural properties, blood coagulation factors can be divided into:
1. Serum enzymes:
- vitamin K-dependent: II, VII, IX, X;
- contact system factors: XI, XII, prekallikrein;
2. Transamidase: XIII. 3. Coagulation cofactor system:
- plasma: V, VIII, high molecular weight kininogen (HMK), fibrinogen;
- fabric: so-called tissue factor (TF).
Conventionally, a distinction is made between external and internal mechanisms of blood coagulation activation (Figure 2).
The blood clotting process can be divided into 3 stages:
A complex of sequential reactions leading to the formation of prothrombinase, or prothrombin activator complex, which includes: factor Xa, platelet factor III (phospholipid), Va and VIIIa factors and Ca2+ ions. This is the most difficult and lengthy phase.
Under the influence of prothrombinase, prothrombin transforms into thrombin.
Under the influence of thrombin, fibrinogen turns into fibrin. Then fibrin stabilizes.
The main component of the extrinsic coagulation pathway is tissue factor (TF). TF is an inner membrane protein synthesized by macrophages and endothelial cells. The synthesis of TF is induced by endotoxins and a number of cytokines. Phospholipids, primarily phosphotidylserine, play an important role in TF activation. TF functions as a cofactor for blood clotting factor VII. Activated factor VIIa, in turn, converts factor X into an active state. The external pathway of blood coagulation occurs much faster than the internal one, and therefore, it can be considered as an “emergency” version of coagulation (5).
The internal pathway of blood coagulation begins with the activation of factor XII.
Factor XIa converts factor IX into IXa in the presence of Ca2+ ions. Activation of factor X is catalyzed by a Ca2+-dependent membrane complex consisting of factors IXa, Va and VIIIa (intrinsic pathway) and/or factors VIIa and TF (extrinsic pathway).
Factors Va and VIIIa are coenzymes for the activation of factor XI.
After the formation of the prothrombin activator complex, the second stage of hemocoagulation begins - the transition of prothrombin (factor II) to its active form - thrombin. It is interesting to note that prothrombin, in addition to its coagulant function, is involved in the differentiation of nerve cells. The transition of prothrombin to thrombin occurs in 2 stages: the formation of mesothrombin and the formation of prothrombin F1+2 fragments. The latter is used to diagnose hypercoagulable states. Thrombin is the end product of the second stage of hemocoagulation; in addition, it causes the activation of cofactors and platelets. Thrombin takes an active part in the reparative processes of damaged tissues.
The formation of fibrin and its stabilization represent the third, final stage of thrombus formation. This process includes 3 phases:
- cleavage of fibrinopeptides from fibrinogen (factor I) under the influence of thrombin;
- fibrin polymerization;
- stabilization of fibrin under the influence of factor XIIIa (fibrin-stabilizing factor).
In the first phase, under the influence of thrombin, fibrinogen is split into fibrinopeptides A and B. Subsequently, soluble complexes of fibrin monomers (RCMF) are formed. These substances are used in practice as a test to determine the degree of fibrin formation activity. In parallel, fibrin polymerization occurs and then fibrin stabilization occurs with the participation of factor XIIIa. It is important to note that factor XIII plays an important role as a matrix that ensures the growth and proliferation of trophoblast and placenta.
The fibrinolytic system is an integral part of the hemostatic system because it always accompanies blood coagulation and is even activated by the same factors as the hemocoagulation process.
Plasma elements, platelets and other cells take part in the process of fibrinolysis. The main enzyme that destroys fibrin is plasmin, which is formed from inactive plasminogen during activation (Figure 3). The process of plasminogen activation includes 3 pathways:
- interior;
- external;
- exogenous.
The exogenous pathway is the main one, but both the intrinsic and exogenous pathways play an important role. The intrinsic fibrinolysis pathway accounts for about 15% of all fibrinolytic activity. Activation of plasminogen through the internal pathway occurs with the participation of factor XII, prekallikrein, high molecular weight kininogen (HMK) and factor XI.
The external pathway of plasminogen activation occurs with the participation of two main activators: tissue (t-PA) and urokinase (u-PA) types.
The exogenous pathway of fibrinolysis activation is associated with bacterial proteins, in particular streptokinase and staphylokinase. At the first stage of fibrinolysis, the X fragment is detached, which is then split into Y and D fragments. The X- and Y-fragments are called “early” or high molecular weight degradation products of fibrin and fibrinogen (FDP). The Y fragment is further broken down into an E fragment and another D fragment. The D- and E-fragments are “late”, or low molecular weight, PDFs (Figure 4). As a result of complete degradation of the fibrin clot, D-dimers (DD) are formed.
PDFs have a pronounced anticoagulant effect. They not only block fibrin, but also prevent the formation of prothrombin and polymerization of fibrin monomers, reduce or suppress the adhesive and aggregation function of platelets. Determining the nature and content of PDF is important in assessing the forms of DIC syndrome, since it determines the extent of intravascular coagulation. It is important to note that PDPs significantly suppress uterine contractility (1).
Natural anticoagulants can be divided into primary and secondary. Primary ones are found in plasma and blood cells and act regardless of whether a blood clot is formed or dissolved. Secondary anticoagulants arise during the process of blood coagulation and fibrinolysis due to the proteolytic action of the enzyme on the substrate.
Antithrombin III (AT III) is the main physiological inhibitor of coagulation factors, capable of blocking prothrombinase by both external and internal mechanisms, including factors XIIa, XIa, VIIIa, IXa, Xa, thrombin and kallikrein. AT III is synthesized in the liver and endothelium of the microvasculature. Heparin increases the degree of inhibition of AT III coagulation factors several thousand times. In addition to AT III, heparin cofactor II (HC II) has inhibitory properties in relation to thrombin exclusively.
A potent physiological anticoagulant is the extrinsic coagulation pathway inhibitor (TFPI), or lipoprotein-associated coagulation inhibitor (LACI). TFPI is mainly synthesized in the endothelium of the microvascular bed, and to a lesser extent by megakaryocytes and fibroblasts. TFPI is a cofactor for low molecular weight heparin (LMWH). LMWH is able to increase TFPI levels in the blood by 500%. TFPI is the most important inhibitor of complex VIIa - tissue factor (VIIa - TF). In addition, TFPI inhibits factor Xa and, to a lesser extent, factor IXa.
Other hemocoagulation inhibitors are C1-esterase inhibitor, α2-macroglobulin, α1-antitrypsin.
Protein C (PC) is synthesized in the liver together with protein S (PS) and thrombomodulin (TM) and is an important regulator of the coagulation cascade, which operates on the principle of negative feedback (Figure 5). To carry out the anticoagulant function of the PC, its activation is necessary, leading to the formation of activated protein C (APC). This process is carried out with the participation of factor Xa, thrombin, thrombomodulin (TM). The main significance of APC is the inactivation of factors V, Va and VIIa, which prevents the generation of the prothrombin activator complex. These reactions are enhanced in the presence of Ca2+ ions, anionic membranes and protein S (PS) (6).
APC also enhances fibrinolysis, which is associated with its ability to neutralize plasminogen activator inhibitor (PAI-1). The anti-inflammatory effect of APC is associated with inhibition of the production of pro-inflammatory cytokines; APC is inhibited by PAI-1, α1-antiplasmin, α2-macroglobulin.
Thrombomodulin (TM) is localized on the surface of the endothelium, performs an anticoagulant function and ensures thromboresistance of the vascular wall. HM has a positive effect on the processes of intrauterine development of the fetus. During the degradation of HM, soluble HM appears in the bloodstream, which is regarded as a marker of endothelial damage and an early preclinical sign of preeclampsia.
In addition to primary natural anticoagulants, secondary anticoagulants are formed during blood clotting. These include PDFs, “spent” ones, i.e. past activation phases, blood clotting factors.
Pathological anticoagulants are absent in the blood under normal conditions, but appear in various immune disorders. These include antibodies to blood clotting factors, most often factors VIII and V (often occurring after childbirth and massive blood transfusions), and immune complexes - lupus anticoagulant.
Thus, the system for regulating the aggregate state of blood (RAS) is subject to the laws of positive and negative feedback, when almost every component of this system, having fulfilled its original function, goes into a state that provides opposite effects. At the same time, according to B.I. Kuznik (7), this system “is configured in such a way as to ensure blood clotting, because there is no condition in which the body would need bleeding.”
Currently, the dominant point of view is that certain conditions are created in the body of a pregnant woman for the development of disseminated intravascular coagulation syndrome. This is expressed in an increase in the total coagulant potential (total activity of coagulation factors), an increase in the functional activity of platelets with a slight decrease in their number, a decrease in fibrinolytic activity with an increase in PDP, a decrease in the activity of AT III with a slight decrease in its content. These features are compensatory and adaptive in nature and are necessary both for the normal formation of the feto-placental complex and for limiting blood loss during childbirth.
Changes in general hemodynamics in the body of a pregnant woman play an important role in the activation of the hemostatic system. For the normal functioning of the feto-placental system in conditions of high coagulation potential of the blood, compensatory and adaptive mechanisms come into play: an increase in the number of small-caliber terminal villi with hyperplasia and peripheral location of capillaries, a decrease in the thickness of the placental barrier with thinning of the syncytium, the formation of syncytiocapillary membranes, syncytial nodules.
Features of the functioning of the hemostasis system are associated with certain changes in the system of spiral arteries of the uterus, such as invasion of trophoblast cells into the wall of spiral arteries, replacement of the internal elastic membrane and internal media with a thick layer of fibrin, disruption of the integrity of the endothelium and exposure of collagen subendothelial structures. In this process, the deployment of the intervillous space with its inherent morphological and hemodynamic features is also important (8).
During a physiologically occurring pregnancy, changes in the hemostatic system occur in proportion to the gestational age. These changes are a physiological adaptation and have 2 main functions - maintaining the normal functioning of the feto-placental complex and stopping bleeding from the placental site after separation of the placenta.
The platelet level during uncomplicated pregnancy remains virtually unchanged. During pregnancy, an increase in all coagulation factors is observed, with the exception of factors XI and XIII. An increase in fibrinogen levels begins from the 3rd month of pregnancy. It is important to note that the fibrinogen content in the peripheral blood is higher than in the uteroplacental bloodstream.
The coagulation potential of the blood also increases due to the fact that the level of antithrombin III decreases. Protein C increases mainly in the postpartum period, while protein S is decreased during pregnancy and significantly decreased after childbirth.
A decrease in fibrinolysis was noted at the end of pregnancy and during childbirth; the concentration of RCMP increased from 8 weeks of pregnancy in parallel with an increase in fibrinogen content. The levels of some fragments of fibrin degradation products increase from 16 weeks of pregnancy and reach a plateau at 36–40 weeks.
Plasminogen levels increase during pregnancy. Tissue plasminogen activator (t-PA) is neutralized by plasminogen activation inhibitors. During pregnancy, the placental type of inhibitor (PAI-2) is of leading importance, the level of which increases by 25 times by the end of pregnancy. The concentration of endothelial inhibitor (PAI-1) increases from 25 weeks of pregnancy. It is believed that PAI-2 plays a role in invasion processes and also has a protective function against premature placental abruption (9).
Diagnostic tests to determine blood viscosity
Often, too thick blood during pregnancy is detected during routine testing. When blood is taken from a finger, it clots too quickly, clogs the tubes, flows poorly, etc.
However, visual analysis alone is not enough to correctly diagnose and identify the causes of pathology. A coagulogram is intended for these purposes. This diagnostic method helps determine whether there are problems with blood clotting and helps develop the correct treatment tactics. The examination is carried out only on an empty stomach.
The following indicators are important for making a diagnosis:
- Prothrombin index. It shows the rate of blood clotting as a percentage. The optimal indicators are 110% with deviations of 32%. If the patient's readings are higher, the blood is too thick.
- Fibrinogen content. In the first trimester, its amount should be from 2 to 4 g/l, in the third – about 6.
- Thrombin time is the rate at which a blood clot forms. Normally, it is formed in 15 s. For pregnant women, the indicator is slightly increased - up to 25 s.
- Lupus coagulant - a woman's tendency to develop lupus.
- Activated partial thromboplastin time. Normally it ranges from 25 to 35 s, for pregnant women - from 17 to 20 s.

Treatment of bleeding disorders during pregnancy.
Coagulation disorders are, even today, one of the most innumerable and most dangerous complications of pregnancy. A condition for effective treatment is knowledge of the physiological changes in hemostasis during pregnancy. The most common are acute acquired disorders of hemostasis, such as loss and consumption coagulopathies associated with significant blood loss and inadequate volume replacement (for example, postpartum atony) and with disseminated intravascular coagulation (PIG) induced by various obstetric pathologies. In 10% of all pregnancies, one should expect thrombocytopenic bleeding of various origins. Von Willebrand disease is the most common congenital bleeding diathesis, which, like very rare hereditary coagulopathies (for example, hemophilia A and B), requires a differentiated analysis of coagulation and an interdisciplinary approach with specific intra and postpartum replacement therapy.
Normal clotting changes during pregnancy
Normal pregnancy is characterized by a state of hypercoagulability, primarily associated with a progressive increase in factors VII (by almost 80%), VIII (by 10%), X (by 80%) and fibrinogen (by almost 70%) from approximately 20 weeks of pregnancy. Prothrombin (factor II), factor V and factor IX show no or very slight increases in concentration during pregnancy, while factor XIII decreases to approximately 50% of normal values before term. The consequence of this in the 3rd trimester is a significant prolongation of prothrombin and thrombin times with a generally unchanged aPTT.
Of clinical importance is the fact that due to pregnancy-induced increases in the production of special coagulation factors (eg, factor VIII), mild forms of congenital coagulopathies may remain clinically undetected. Coagulation inhibitors behave differently during pregnancy: antithrombin III basically does not change with a slight decrease until the end of pregnancy, as does protein C, and vice versa, the activity of total protein S gives a significant decrease. In addition, during pregnancy there is a decrease in fibrinolytic activity (for example, an increase in plasminogen activator inhibitor).
Increased platelet aggregability in late pregnancy should rather be considered as a factor promoting thrombosis, while the number of platelets and platelet survival time during physiological pregnancy generally remain unchanged. Detailed reviews on the topic can also be found (16,18).
In general, during normal pregnancy there is a Low-grade activation of coagulation, recognized among other things by an increase in the concentration of D-dimers (terminal lysis product of reticular fibrin), which is functionally compensated by pregnancy-induced hemodilution with an increase in capillary perfusion and microcirculation.
| Table 1 | |||
| Critical breakpoints for acute bleeding complications | |||
| Parameter | Borderline value | ||
| Hemoglobin | <8.5 g/l for persistent bleeding | ||
| Platelet count | 50.000/mg | ||
| Quick value | <40% | ||
| aPTT | >1.5 times the normal value | ||
| Fibrinogen | <100 mg/dl | ||
| table 2 | |||
| Therapy for dilutional coagulopathy | |||
| Elimination of the cause of bleeding (surgically or medically) | |||
| With a loss of up to 20% of blood volume (1.2 l): crystalline/colloidal solution iv (eg HAES) | |||
| If there is a loss of > 20 (-30)% of blood volume Red blood cell concentrate Frozen Plasma (FFP) | 2(-3) 1 | ||
| In an emergency: red blood cell concentrate FFP | 0 Rhesus negativ AB blood type | ||
| In the absence of FFP: fibrinogen concentrate antithrombin-III concentrate | 50 mg/kg body weight 25 IE/kg body weight | ||
| Platelets <30,000 (50,000)/ml: platelet concentrate | |||
| Table 3 | |||
| Treatment of severe therapy-refractory hemostasis disorders with recombinant activated factor VII (Novo Seven) | |||
| Mechanism of action | Isolation and binding of "tissue factor" to factor VIIa ® factor VIIa activates on the surface activated platelets directly factor X ® massive thrombin formation (“Thrombin-Burst”) ® formation of a stable fibrin clot | ||
| Observation | No special diagnosis, clinical effect (bleeding) | ||
| Dosage | 90 mg kg body weight, repeat after 15 minutes | ||
| Used as Ultima ratio - effectiveness in case descriptions. Doesn't have permission. | |||
Acutely acquired hemostasis disorders
Coagulopathies of loss and consumption
The common end stage of untreated peripartum bleeding of various origins is loss or dilution coagulopathy and/or consumption coagulopathy. During pregnancy, due to an increase in circulating blood volume by an average of 1.5-1.7 times, “protective hypervolemia” develops, which, in conjunction with the rapid postpartum mobilization of a significant volume of venous blood, as well as through the elimination of Vena-cava-inferior compression after childbirth at the beginning can compensate for varying and individually high blood loss. The basic rule is that a pregnant woman's blood volume is 8.5-9% of her body weight.
With a loss of >20% (1.2-1.5 l) of circulating blood, the damping capabilities are not enough. The necessary initial volume injections, although they can prevent the development of shock with centralization, nevertheless, they lead to a strong dilution of the hemostatic potential, which is not enough for effective hemostasis (loss-or-dilution coagulopathy). Critical breakpoints for acute bleeding complications in relation to overt coagulopathy are presented in Table 1.
The main causes of this coagulopathy loss are:
- postpartum uterine atony (75-82% of all postpartum bleeding complications),
- severe obstetric injuries,
- placental abruption disorders,
- premature placental abruption (often a combination of disseminated intravascular coagulation/losing coagulopathy).
Rare causes include bleeding during abortion and rupture of an ectopic pregnancy and bleeding due to Plazenta-praevia. The emergency obstetric approach in these situations has been described in detail in recent reviews (16,17).
Therapy
The parameters that determine the therapeutic approach are the severity of bleeding (actually blood loss), the clinical condition of the pregnant woman and the results of the blood picture and coagulation analysis (Table 1). Together with the rapid elimination of the cause of bleeding (medication, surgery), with an initial blood loss of up to 20% of the blood volume, a quick replacement of the volume is most often sufficient: 1 ml of blood loss per 3 ml of crystalline solution. Blood losses exceeding 20-30% of blood volume require rapid administration of red blood cell concentrates and fresh frozen plasma, which contains all coagulation proteins, including antithrombin III in a physiological combination: initially in a ratio of 2:1 or 3:1 (table 2).
In case of persistent bleeding, a hemoglobin value of 8.5 mg/dl is a therapeutic indicator for starting replacement with red blood cell concentrates. If prophylactic replacement therapy has not been carried out and, based on an early determination of global coagulation, a decrease in clotting potential (<100 mg/dl) that is significant for hemostasis is determined, then freshly frozen plasma corresponding to the blood group is immediately administered.
If, with severe and persistent bleeding, the necessary frozen plasma is not available (it must be thawed first) or, despite the introduction of FFP, an unacceptable decrease in the breakpoint values occurs (Table 1), then it is recommended to replace clotting factor concentrates with concentrates (Table 2): for example, if the fibrinogen level is <100 mg/dl, replacement with fibrinogen is carried out at a dose of 30-50 mg/kg body weight. If possible, maintain fibrinogen levels > 100 mg/dl (Cave: risk of thrombosis with significant replacement!).
A new promising and, in some cases, life-saving treatment is the intravenous administration of recombinant activated factor VII (Novo Seven®): dosage 60-90 g/kg body weight, repeated after 10-15 minutes if necessary (Table 3). This begins the coagulation process through the release and binding of “tissue factor” to activated factor VII. Factor VIIa directly activates factor X on the surface of activated platelets and thus causes massive thrombin formation, the so-called “Thrombin-burst”, which quickly leads to the formation of a stable fibrin clot.
Laboratory and chemical monitoring of this therapy is not required; the indicator is a decrease in the severity of bleeding. Recombined activated factor VII is currently only approved for the treatment of Hemmkorper hemophilia, but in the future - not least thanks to many descriptions of successful cases (see review) - it will undoubtedly take, at least as an Ultima ratio for surgical interventions, a strong place in treatment of life-threatening obstetric complications of bleeding (clotting disorders).
The optimal time for administration of platelet concentrate is not certain. Even if, with thrombocytopenia < 20,000/l, we have to count on the occurrence of spontaneous bleeding, we decide to administer platelet concentrate, especially before surgical revision due to subsequent bleeding (for example, after Sectio caesarea) already when the platelet count drops <50,000/ l. After consolidation of the coagulation situation (fibrinogen > 200 mg/dl), platelet count >100,000/l, it is recommended after severe peripartum bleeding complications to carry out drug prophylaxis of thrombosis, preferably with low molecular weight gerin.
Disseminated intravascular coagulation and consumptive coagulopathy
This systemic thrombohemorrhagic complication is characterized by the following clinical phases:
- activation of the coagulation system (hypercoagulation),
- disseminated intravascular coagulation with consumption of coagulation factors and inhibitors, including reactive hyperfibrinolysis,
- development of consumption coagulopathy with bleeding and/or micro-/macrothrombosis,
- organ failure with diffuse hemorrhages.
The obstetric pathologies leading to disseminated intravascular coagulation (DIG) are presented in Table 4, and the pathophysiology and diagnosis are described in detail elsewhere (16). Of clinical significance is the fact that the stage of activation of intravascular coagulation (recognized by a sharp jump in D-dimers) can pass through the phase of intravascular fibrin formation, which is not yet clinically noted and often not determined by global coagulation parameters, gradually and imperceptibly over several hours into a clinically obvious coagulopathy, Because of this, if there is appropriate suspicion, it is necessary to carry out routine laboratory chemical control first after 1-2 hours. Diagnosis of DIG is presented in Table 5.
Therapy
The condition for successful treatment is the correct diagnosis and elimination of the underlying pathology, usually by immediate termination of pregnancy (sometimes Sectio caesarea) for example, in severe premature detachment and/or fulminant HELLP syndrome.
Along with balanced volume replacement, timely administration of red blood cell concentrates and fresh frozen plasma is decisive to maintain adequate microcirculation (Table 6).
| Table 4 | ||
| Causes of disseminated intravascular coagulation and consumptive coagulopathy | ||
| Premature placental abruption | ||
| Amniotic fluid embolism | ||
| Septic conditions (chorioamnionitis, puerperal sepsis) | ||
| Severe hypertensive complications of pregnancy (preeclampsia, eclampsia, HELLP syndrome) | ||
| Intrauterine fetal death (Dead-fetus-Syndrome) | ||
| Extensive tissue trauma | ||
| Table 5 | ||
| Diagnostic criteria for disseminated intravascular coagulation (15 each) | ||
| Parameter | Critical value | Trend without treatment |
| Platelets | <100.000/ml | Demotion |
| Quick indicator | <50% | Demotion |
| aPTT | >1.5 times | Increase |
| Fibrinogen | <100 mg/dl | Demotion |
| Antithrombin | <50% | Demotion |
| D-dimers | >600 ng/dl | Increase |
In this case, the administration of FFP is the basis of therapy: according to our own experience and in accordance with the guidelines of Bundesarztekammer (2001), in this situation, thanks to the timely administration of at least 2 units of frozen plasma, coagulopathy can be avoided, and above all when, during Sectio caesarea, it is already signs of decreased blood clotting ability appeared. It should be taken into account that at this moment the parameters of general coagulation are changed slightly or are within normal limits or have not yet been analyzed. In some cases, antithrombin deficiencies that cannot be corrected with frozen plasma should be corrected (Table 6).
When using PPSB drugs (containing vitamin K-activated coagulation factors II, VII, IX, X, as well as proteins C and S), caution should be exercised, since these drugs may contain varying amounts of already activated coagulation factors with a subsequent risk of developing severe thromboembolic complications. Therefore, administration of antithrombin is necessary before use. For the administration of platelet concentrates, the same values (scales) apply as for loss coagulopathy.
There is still ongoing debate about the use of heparin in relation to DIG and obstetric complications (mainly DIG syndrome). More recently, reports of severe postpartum bleeding complications with heparin use in postpartum HELLP syndrome have resurfaced. Without going into the details of this discussion (see review 16), it should be emphasized that heparin (even in low dosages), until bleeding has stopped or there is an increased risk of bleeding, should never be used in therapy acute obstetric consumption coagulopathies. This especially applies to HELLP syndrome, severe preeclampsia and premature placental abruption! Heparin is not used in the treatment of acute obstetric consumptive caogulopathies.
Extremely rarely, excessive fibrinolysis and fibrinogenolysis can cause severe postpartum coagulation disorders (for example, after amniotic fluid embolism, the incidence of which is 1: 6000-80,000 of all births). In these cases, if bleeding persists despite the above measures, after or during replacement treatment, the use of antifibrinolytics is indicated: for example, the polyvalent proteinase inhibitor Aprotinin (Trasylol) at an initial dose of 500,000 KIE (Kallikrein-Inhibitor-Einheiten) intravenously, followed by a subsequent dose of 200,000 KIE after 4-6 hours or until bleeding stops. Also, in cases of severe treatment-refractory consumption coagulopathy due to obstetric pathology, descriptions of the successful use of recombinant activated factor VII have again appeared, so this Ultima-ratio option should always be kept in mind.
| Table 6 |
| Treatment of acute hemostasis disorder |
| Volume replacement (ZVD, ureter, hemoglobin, hematocrit) ® maintenance of microcirculation ® correction of metabolic acidosis |
| Fresh frozen plasma (FFP) and red blood cell concentrates According to circumstances 2 FFP “preventatively” |
| Persistent bleeding (FFP insufficient or absent) Antithrombin-III concentrate: 25 IE kg body weight Quick value <40%: PPSB preparation (25 IE kg body weight) Fibrinogen <100 mg/dl: fibrinogen concentrate (50 mg/kg body weight) |
| For excessive fibrinolysis and life-threatening bleeding post partum Aprotinin (eg Trasylol) |
| No heparin while bleeding continues or there is an increased risk of bleeding! |
Chronic acquired disorders of hemostasis
These include primarily platelet hemorrhagic diathesis:
- autoimmune thrombocytopenia (ATP, ITP),
- thrombotic-thrombocytopenic purpura (TTP),
- drug-induced thrombocytopathies (review 18).
Caused by hemodilution and/or platelet breakdown, 4-8% of pregnancies develop what is called “gestational thrombocytopenia.” Clinically defining is a platelet count <70,000/l without additional tendency to bleeding, even during labor. However, these pregnant women with gestational thrombocytopenia have a 7.4 times higher risk of developing HELLP syndrome in subsequent pregnancies.
Autoimmune thrombocytopenia
Idiopathic thrombocytopenic purpura (ITP) is defined as isolated thrombocytopenia without clinically apparent comorbidities or other causes of low platelet count (eg, lupus, HIV, medications) and is the most common cause of thrombocytopenia in the 1st and 2nd trimesters. Clinically, petechial hemorrhages on the skin and mucous membranes come to the fore. An overview of diagnostics can be found in review (1). There is a slightly increased risk of postpartum bleeding complications, and attention should be paid to optimal surgical control of bleeding in obstetric wounds.
Therapy
Therapy depends on platelet concentration. Platelet count >30,000/l without bleeding generally does not require any therapy. However, according to the conditions for the prevention of bleeding, the platelet count during childbirth and when using regional anesthesia should be in the range of >50,000/l. The absolute indication for treatment is thrombocytopenia <10,000/l, regardless of the duration of pregnancy, as well as in pregnancies in the 2nd and 3rd trimesters with a platelet count between 10,000 - 30,000/l.
The first choice methods are the administration of glucocorticoids (prednisone at an initial dose of 1 mg/kg body weight per day for 2-4 weeks) and the use of immunoglobulins (1 g/kg body weight initially and repeated after 2 days). The latter is indicated if >10 mg prednisone per day is required to maintain platelet counts >30,000/l. Since the rise of platelets occurs quickly (within 6 hours), it is rational to use immunoglobulins shortly before childbirth. If resistance to therapy appears, combination therapy with glucocorticoids (for example, methylprednisolone 1 g/day for 3 days) and immunoglobulins is indicated.
Due to the transplacental passage of maternal IgG antibodies, 10-25% of newborns develop thrombocytopenia <50,000/l and in 5% of cases <20,000/l; in any case, intracranial hemorrhages are extremely rare. The obstetric approach to ITP is described in detail elsewhere (1, 11).
Thrombotic thrombocytopenic purpura
Thrombotic thrombocytopenic purpura (TTP, Moschkowitz-Syndrome) is a thrombotic microangiopathy secondary to epithelial damage with the classic symptoms of severe Coombs-negative microangiopathic hemolytic anemia, thrombocytopenia, fever in 60% of patients, neurological symptoms (seizures, transient hemiparesis), and dysfunction kidneys without hematological effects on the fetus.
Therapy
The treatment of choice is plasma exchange transfusion, which is likely due to the removal of high molecular weight von Willebrand multimers. This involves replacing the patient's plasma with fresh frozen plasma (FFP). Initially, 30-40 ml of patient plasma per kg body weight should be removed. Reducing this withdrawal volume to 15-20 ml/kg body weight is only justified if the platelet count increases >30,000/l or if neurological and renal symptoms improve. Treatment can mainly be carried out during pregnancy and gives a cure rate of up to 80%. Optimal interdisciplinary collaboration with an experienced hematologist is important. The administration of platelet concentrates is contraindicated, and the use of glucocorticoids is useless (review 8, 16).
Congenital disorders of hemostasis
von Willebrand's disease
It is an autosomal dominantly inherited multifactorial disorder with different subtypes and a prevalence of 0.8%. During pregnancy and post partum, bleeding in 1-3% is associated with a deficiency of von Willebrand factor (vWF). Diagnostic indicators include increased bleeding time, decreased activity of factor VIII (FVII-IC and vWF), and often prolonged PPT. Clinically, the patient has a typical history of bleeding after trauma, menorrhagia, or hemorrhage after surgery.
Since during normal pregnancy there is an increase in factor VI-II complex, bleeding complications during pregnancy are rather rare (bleeding times are often normal). Of particular importance are the bleeding complications that arise immediately post partum or on the 7-10th day after birth, which may be the first signs of von Willebrand's disease.
Therapy
Pregnant women who at the time of delivery have factor VIIC concentrations >50U/dl and are asymptomatic do not require any therapy (up to 80% type I, decreased vWF concentrations). At levels <30 U/dl, vWF concentrates should be administered antepartum and post partum therapy should continue with Desmopressin (Minrin® parenteral or nasal spray) (Table 7). In type IIb (excessive response to Ristocitin), Desmopressin is relatively contraindicated, as existing thrombocytopenia may otherwise be aggravated.
| Table 7 | ||
| Therapy of von Willebrand disease during pregnancy and post partum | ||
| Type | Degree | Therapy |
| 1 | Weak (vWF>30 IU/dl | Desmopressin 0.3 mg/kg weight in 50 ml 0.9% NaCl/30 min., repeat after 12-24 hours, if there is no effect, then replacement: first 40-60 IU/kg weight vWF then 40-50 IU/ kg body weight |
| 1 | Moderate (vWF<30 IU/dl) | 40-50 IU/kg (1 or 2 doses) or every 8-12 hours for 3 days, up to vWf >50 IU/dl |
| 2 or 3 | Severe (vWF < 30 IU/dl) | initially 40-60 IU/kg weight then 40-60 IU/kg weight every 8-12 hours for 3 days until vWF > 50 IU/dl |
| vWF: von Willebrand factor | ||
In this situation and in the presence of type III, it is necessary to administer plasma concentrates (for example, recombinant factor VIII-vWF) post partum and in the postpartum period. The following dosage is recommended:
- 30-40 IU/kg body weight initial dose,
- 15-25 IU/kg weight every 12 hours for the first 3-5 days,
- 15-25 IU/kg weight every 24 hours for a further 5-7 days.
At critically low platelet values (<20,000/l), the administration of platelet concentrates can be considered under the protection of the replaced vWF. There is still no evidence that, due to the potential risk of hemorrhage in the fetus or newborn, Sectio caesarea has advantages over vaginal delivery.
Congenital condition of lack of factors
An up-to-date review of extremely rare inherited bleeding disorders due to deficiency of clotting factors I, II, V, VII, X and XI and XIII was recently published by Bolton-Maggs et al. These congenital deficiency conditions have a frequency of 1:1-2 million and are therefore not considered here.
Along with vWF deficiency, the most common are X-chromosomal recessive factor VIII deficiency (hemophilia A) and factor IX deficiency (hemophilia B) with a frequency of 1-2/10,000 to 1-2/100,000.
| Table 8 | |
| Boundary values of coagulation factors of normal hemostasis | |
| Coagulation factors | Limit values |
| Fibrinogen | 100 mg/dl |
| Factor II, V, VII, VIII, IX, X | 30-40% |
| Factor XI | 20% |
| Factor XII | 1% |
For clinical everyday practice, the concentration limit values given in Table 8 are defining and guiding. During pregnancy, a woman with hemophilia experiences a significant increase in factor VIII, while factor IX does not show any significant changes. If only one chromosome is affected, then with hemophilia, most often only a drop in the concentration of factors occurs by 50% of the norm (sufficient for hemostasis); 10-20% of women are carriers, but activity is defined as <40%, so there is a significant risk of bleeding.
Determination of factors should be carried out between 28 and 32 weeks of pregnancy and, if possible, at birth, including coagulation testing. If actual analysis of sub partu factors is not possible, then the latest result can be taken from the 3rd trimester. At levels <50 IU/dl, adequate substitution should be carried out. The required replacement requirement for factor VIII/IX with highly purified concentrates is calculated using the formula where one unit per kg of weight increases the measured activity by 1-2%. If in a real situation there is no concentrate of factors available, then in exceptional cases the use of PPSB is justified.
The incidence of cerebral hemorrhages in hemophilic newborns is 1-4%, the magnitude of severe cerebral hemorrhages during vaginal delivery is quite small, and therefore Sectio caesarea is not justified. Spinal/epidural anesthesia with factor concentrations >50 IU and normal general coagulation is possible.
Summary for practice
Treatment of bleeding disorders during pregnancy and, above all, sub partu, should be mastered by every practicing obstetrician, since it largely determines emergency management, on the success of which the fate of the affected woman depends. This applies primarily to the timely treatment of loss and consumption coagulopathy, in which the availability of a laboratory and the timely use of blood components and frozen plasma are an absolute prerequisite for every obstetric activity.
Chronic acquired disorders of hemostasis, both idiopathic and thrombotic thrombocytopenic purpura (ITP, TTP), also require, as well as adequate and type-appropriate therapy for von Willebrand disease and as therapy for congenital conditions of factor deficiency, strong interdisciplinary cooperation with differentiated coagulation analysis and specific replacement therapy intra and post partum.
Source: W. Rath, L. Heilmann. Therapie von Gerinnungsstörungen in der Schwangerschaft. Gynäkologe 2005. 38:791-798
Translation from German – Yu.M. Bogdanov, Department of Pediatrics, Faculty of Education, Northern Medical University, Arkhangelsk