General information
Antigen CA 72-4 is a high molecular weight glycoprotein that is produced by almost all tissues in the fetal body. In an adult, its production is completely stopped, so normally the antigen in the blood is either not detected at all, or its amount is negligible.
An increase in tumor marker values is observed only in the presence of malignant neoplasms of glandular origin (gastric carcinoma, colorectal cancer, mucinous ovarian tumors) that actively produce CA 72-4. An increase in antigen concentration is also observed in tumors of other localizations (lung, breast, pancreas cancer).
An increase in CA 72-4 values is observed
- for cancer of the gastrointestinal tract in 40% of cases,
- for lung cancer – 36%,
- for ovarian cancer – 24%.
Benign pathologies of the digestive organs, ovaries, lungs, etc. also stimulate the expression of CA 72-4. Therefore, the analysis for this tumor marker cannot be called specific. Yes, based on its results one can judge the presence of a malignant process in the body, but it is not possible to accurately determine the location and type of tumor. To obtain the most complete information, analysis for CA 72-4 is almost always combined with other studies: CEA or CA 19-9 (for monitoring stomach cancer), CA 125 (for monitoring the course and treatment of ovarian cancer).
A case of an increase in CA 72-4 due to Helicobacter pylori infection in a 66-year-old patient is described, and the level reached values similar to those for gastric carcinoma. CA 72-4 slowly returned to normal levels after two courses of eradication therapy (treatment aimed at killing Helicobacter pylory).
What do tumor markers show for stomach cancer?
In general, tests for tumor markers for stomach cancer are not highly sensitive or specific. This means that normal results do not guarantee the absence of a tumor, and elevated results do not mean the presence of cancer. However, when the tumor marker level increases tenfold, the likelihood of this increases.
The diagnostic value of the study of tumor markers increases with the simultaneous study of several indicators, for example, CA 19-9 and CEA, or CA 72-4 and CEA. But they still cannot be used for self-diagnosis of stomach cancer.
SA 19-9
Reference values for the CA19-9 tumor marker are in the range of 0-34 U/ml. Such indicators are observed in 95% of healthy people.
An increase in tumor marker levels may indicate the following diseases:
- Pancreas cancer. The higher the level of CA 19-9, the more widespread the pathological process. The highest concentration is observed in the presence of distant metastases, i.e. at the 4th stage of the disease.
- Stomach cancer.
- Colon cancer.
- Liver carcinoma.
- Cancer of the bile ducts and gallbladder.
- Ovarian cancer.
In addition, an increase in the level of this tumor marker is observed in some benign diseases:
- Hepatitis.
- Cirrhosis of the liver.
- Pancreatitis.
- Cholelithiasis.
- Cystic fibrosis.
REA
The REA reference values are as follows:
- For non-smokers below 3.8 ng/ml.
- For smokers - 0-5.5 ng/ml.
These are normal levels of CEA, characteristic of healthy people, but this result is also possible in the presence of a tumor that is insensitive to this test.
An increase in the level of this tumor marker can be observed in both malignant and benign diseases. Malignant tumors:
- Colon cancer.
- Stomach cancer.
- Lungs' cancer.
- Breast and pancreatic cancer.
Non-oncological diseases:
- Cirrhosis of the liver.
- Hepatitis.
- Intestinal polyps.
- Chronic inflammatory bowel diseases, such as ulcerative colitis.
- Some lung diseases, including tuberculosis.
- Autoimmune pathologies.
SA 72-4
Reference values for tumor marker CA 72-4 0-6.9 U/ml. A normal analysis indicator is typical for healthy people; an increase in its value is observed in the following cases:
- Stomach cancer.
- Some forms of ovarian cancer.
- Colorectal cancer.
- Lungs' cancer.
- Hepatitis.
- Cirrhosis of the liver.
- Ovarian cyst.
- Inflammation of the gastrointestinal tract.
Indications for determination of CA 72-4
- Monitoring the condition of patients diagnosed with gastric cancer, monitoring the effectiveness of treatment, predicting relapses;
- Assessment of the severity of the malignant process in gastric cancer, detection of metastases;
- Monitoring the course and treatment of mucinous ovarian cancer;
- Differential diagnosis of malignant and benign neoplasms in the ovaries.
- In combination with other tumor markers for suspected lung and colorectal cancer.
Doctors: general practitioner, oncologist, gynecologist, surgeon, gastroenterologist, proctologist refer for testing and interpret the results of the analysis for CA 72-4.
Tumor markers
Tumor markers
Recently, there have been advances in early diagnosis and treatment effectiveness in oncology. Some types of tumors, if diagnosed early, can be cured in 100% of cases. Determination of unique proteins - tumor markers, which are produced by cancer cells, has been one of the methods for diagnosing tumors for many years.
Analyzes for tumor markers are grouped into certain algorithms that make it possible to identify problems in individual organs, identify the tumor process in the early stages, and also draw distinctions between benign and malignant processes. Thus, if you suspect the presence of rectal cancer, it is necessary to conduct tests for tumor markers CEA and CA-19-9. The same tumor markers are examined for suspected pancreatic cancer. To diagnose stomach cancer, tests are performed for the tumor markers CEA, CA-72-4 and CA-19-9. If breast cancer is suspected, tumor markers CEA and CA-15-3 must be examined. For small cell lung cancer, the algorithm requires tests for tumor markers CEA, CA-72-4, CA-15-3, Cyfra -21-1. Testicular germ cell tumors are based on the study of tumor markers CA-19-9 and beta-hCG, and ovarian tumors are based on CA-125, CA-19-9, beta-hCG and HE4. Thyroid cancer is diagnosed using onomarkers CEA and TG (thyroglobulin). To detect prostate cancer, it is necessary to examine total and free PSA, as well as their ratio, etc.
Tumor marker PSA (Prostate-specific antigen)
Prostate cancer in the early stages is hidden and has no clinical manifestations in the vast majority of patients. Therefore, the primary task during this period is the targeted use of examination methods that allow diagnosing prostate cancer at an early stage . Determination of prostate-specific antigen ( PSA ) , digital rectal examination and transrectal ultrasound examination are not burdensome for the patient and provide an accurate diagnosis of prostate cancer in 80% of cases. In this regard, recently determination of PSA level is increasingly used in outpatient practice and is prescribed not only by urologists, but also by doctors of other specialties
Prostate-specific antigen ( PSA , Prostate-specific Antigen, PSA ) is a tumor marker that is used in the diagnosis of early stages of prostate cancer , as well as in assessing the prevalence, prognosis and effectiveness of treatment. The widespread use of this study has made it possible to increase the detection of prostate cancer at earlier stages, when radical (curative) treatment methods are possible.
Unfortunately, today in Russia no more than 5% of men are aware of the need to undergo preventive examinations and examinations in the absence of any complaints or manifestations of the disease. Thus, in Russia, more than half of men with newly diagnosed prostate cancer have distant metastases, which sharply worsens the prognosis of the disease. For example, in Australia, the widespread use of early diagnostic methods and, accordingly, more effective treatment of prostate cancer has made it possible to reduce mortality from this disease by more than 5 times.
Indicators of prostate-specific antigen ( PSA )
| Interpretation of prostate-specific antigen ( PSA ) | PSA ) level |
| Norm | 0-4 ng/ml |
| Suspicion of prostate cancer | 4-10 ng/ml |
| High risk of prostate cancer | 10-20 ng/ml |
| Risk of advanced prostate cancer | 20-50 ng/ml |
| High risk of prostate cancer | 50-100 ng/ml |
| Prostate cancer accompanied by metastasis | > 100 ng/ml |
Prostate-specific antigen ( PSA ) is produced by epithelial cells of the prostate gland and its increase is possible in other diseases - prostate adenoma (benign prostatic hyperplasia) and acute and chronic prostatitis. Therefore, prostate-specific antigen ( PSA ) is not exclusively a cancer-specific marker and an increase in its level only requires a prostate biopsy.
In addition, an increase in prostate-specific antigen ( PSA ) may be due to the following reasons:
- after prostate massage;
- after sexual intercourse
- after prostate biopsy
Free PSA . Determining this indicator allows you to increase the specificity of the study. The ratio of free and total PSA. The ratio value is given automatically in the laboratory's response if both tests were ordered. In the presence of prostate carcinoma, PSA is present in the serum, mainly in bound form. Therefore, the ratio of free to total PSA decreases and is usually less than 15%.
| Carcinoembryonic antigen |
One of the most important tumor markers is carcinoembryonic antigen (CEA)
Indications for the study:
- Evaluation of the effectiveness of treatment for carcinoma of the colon, rectum, pancreatic, liver, and uterine cancer.
- Prognosis of disease development in colon and rectal carcinoma.
- Evaluation of the effectiveness of treatment for breast carcinoma.
- Differential diagnosis of ovarian tumors.
- Evaluation of the effectiveness of lung cancer treatment.
Combinations of CEA determination with identification of other tumor markers should be used according to the suspected type of cancer. (see picture)
In all patients with cancer of the colon, rectum, stomach and pancreas in the presence of metastases, the concentration of CEA in the blood serum exceeds the norm by at least 2 times. In this case, the degree of increase in CEA concentration depends on the location of metastases.
An increase in the content of CEA in pleural exudate and ascitic fluid was found in 25–40% of cancer patients, more often in transudative than in exudative processes. In 20–25% of patients with malignant neoplasms, an increase in the concentration of CEA in the pleural exudate is the only sign of the presence of a tumor. Higher levels of CEA in the blood serum are observed in 73% of patients with colon cancer, 92% of patients with pancreatic cancer, 57% of patients with liver cancer, 72% of patients with lung cancer, 52% of patients with breast cancer, 53% of patients with uterine cancer and 36% patients with ovarian cancer.
The concentration of CEA in the blood serum of patients with malignant neoplasms of certain localizations (cancer of the alimentary canal, breast) correlates with the degree of tumor invasion and metastasis. The amount of CEA exceeding 80 ng/ml indicates the presence of a metastatic tumor in the body.
2 months after surgery in patients with cancer of the stomach, rectum and colon, the level of CEA concentration decreases to normal, if the operation was radical and there were no distant metastases. With further observation, an increase in CEA levels in such patients indicates the development of distant metastases or relapse. The test for CEA in this regard has increased sensitivity: an increase in the level of CEA occurs 3-6 months before the clinical manifestation of metastases. Such monitoring of patients is very important for prescribing timely reoperation or the use of other types of therapy. A 2-fold increase in CEA can be considered a clinically significant indicator of the onset of relapse in radically operated patients with colon and rectal cancer.
.
Tumor marker Ca 15-3
– a tumor marker used to monitor the course and evaluate the effectiveness of therapy for breast carcinoma.
Found predominantly in the mammary glands, it is also produced by carcinomas of the lungs, ovaries and gastrointestinal tract, including the pancreas. Up to 80% of women with metastatic breast cancer have a significant increase in CA 15-3 levels, while in women diagnosed with stage I-II breast cancer this is observed in 20% of cases. The test is most useful in combination with a CEA study.
In addition to increased production by tumor tissue, increased concentrations of this marker can be observed in other pathological conditions accompanied by increased cell turnover. Since, like other glycoproteins, CA 15-3 is excreted from the blood by the liver, an increase in its level can be detected in liver pathology, especially in cirrhosis,
The dynamics of the marker level is of more interest than a single level value taken by itself. The rate of increase in the tumor marker usually allows one to draw a conclusion about the nature of the progressive course of the disease, in particular, about metastasis. In case of relapses or metastases, the increase in the concentration of CA 15-3 may precede the appearance of clinical symptoms by 6-9 months.
Increasing values:
Oncopathology:
- Breast carcinoma (a particularly high level is observed in late stages and in the presence of metastases).
- Bronchogenic carcinoma.
- Stomach cancer.
- Liver cancer.
- Pancreas cancer.
- Cancer of the ovaries, endometrium, uterus (late stages of tumor development).
Benign processes:
- Benign diseases of the mammary glands.
- Liver cirrhosis (up to 50 U/ml).
- A physiological increase in CA 15-3 during pregnancy in the 3rd trimester is possible - up to 50 U/ml.
- Autoimmune diseases.
Tumor marker for ovarian cancer – CA 125
CA 125 is present in normal endometrial tissue and in the serous and mucinous fluid of the uterus. It does not enter the bloodstream unless natural barriers are broken down. Serum levels may double during menstruation, especially with endometriosis. There may be a physiological increase in the marker during pregnancy (1st trimester). In patients with stage I ovarian cancer, the content of the marker is practically no different from the control, but in stages II, III and IV of the disease, the levels of CA-125 increase significantly. Patients whose CA-125 level decreases in the first 3 months after the start of treatment survive significantly better. In complete remission in the absence of a tumor, the level of CA-125 is close to zero. An increase in CA-125 from zero to 35 U/ml, i.e. within normal limits, may be a preclinical manifestation of a relapse.
An increase in the marker level during remission should be the basis for an in-depth examination of the patient to detect a relapse of the disease. A persistent increase in CA-125 values indicates tumor progression and poor response to treatment.
This test has low specificity; according to statistics, a twofold increase in the level of CA 125 in the blood, especially in women over 55 years of age, is a reliable indication of the presence of ovarian cancer. In ovarian and endometrial cancer, a decrease in marker levels indicates a favorable prognosis and a good response to treatment.
Preparation: on an empty stomach. At least 8 hours pass between the last meal and the blood draw (preferably at least 12 hours). Juice, tea, coffee (especially with sugar) are not allowed. You can drink water.
Increased Ca-125 levels:
oncopathology
- ovarian cancer (in 80% of cases);
- uterine cancer;
- endometrial cancer;
- fallopian tube cancer;
- mammary cancer;
- pancreas cancer;
- rectal cancer;
- stomach cancer;
- lungs' cancer;
- liver cancer.
somatic pathology (slight increase):
- cystic formations of the ovaries;
- endometriosis;
- inflammatory diseases of the appendages, common gynecological infection;
- peritonitis, pleurisy;
- chronic hepatitis, liver cirrhosis;
- chronic pancreatitis;
- autoimmune pathology.
Tumor marker of the gastrointestinal tract Ca-19-9
For health problems, CA-19-9 is produced by pancreatic tumor or and stomach tumor . CA-19-9 is primarily a tumor marker for stomach and pancreatic cancer.
A slight (maximum up to 500 U/ml) increase in CA-19-9 may indicate diseases such as:
- hepatitis
- cholelithiasis
- cholecystitis
- cirrhosis of the liver
- other inflammatory diseases of the gastrointestinal tract and liver
- cystic fibrosis.
A significant increase in the level of CA-19-9 is a sign of serious cancer:
- pancreas cancer
- cancer of the gallbladder or biliary tract and primary liver cancer
- stomach cancer
- colon and rectal cancer
Almost all patients with very high CA-19-9 levels (above 10,000 U/ml) have distant metastases.
After the tumor marker CEA, CA-19-9 is the second most important marker of pancreatic carcinoma. It can also be used to make an accurate diagnosis if carcinoma and pancreatitis are suspected at the same time. most in demand as a method of monitoring the effectiveness of behavioral treatment. CA-19-9 is also used to determine colon carcinoma when the CEA test result is negative.
The tumor marker CA-19-9 is excreted in bile. Therefore, even with the slightest disturbance in the synthesis, secretion and outflow of bile, the level of CA-19-9 can be significantly increased. To avoid unreliable results, a biochemical blood test is simultaneously prescribed: GGT (γ-glutamyltransferase) and alkaline phosphatase.
β2-Microglobulin
β2-microglobulin is normally detected in biological fluids only in small quantities. β2-microglobulin testing is recommended to confirm the diagnosis and monitor patients with multiple myeloma or non-Hodgkin's lymphoma. The increase in marker concentration depends on the stage of the disease, the degree of malignancy and the cell type. In patients with progressive pathology, the concentration of β2-microglobulin is significantly higher than in patients during the stabilization period. High protein levels correlate with poor prognosis. In addition, in patients with chronic lymphocytic leukemia, there is a correlation between the number of peripheral blood lymphocytes and the concentration of β2-microglobulin.
In patients with leukemia, an increase in the level of β2-microglobulin in the cerebrospinal fluid indicates involvement of the central nervous system in the pathological process.
Since the level of β2-microglobulin increases in various autoimmune diseases, disorders of cellular immunity, including AIDS and after organ transplantation, this test is also applicable for monitoring the condition of such patients.
Tumor marker CA 72-4
Normally, it is practically not expressed by healthy tissues in adults. Increased concentrations of CA 72-4 are observed in 40% of gastrointestinal cancer cases, 36% of lung cancer cases, and 24% of ovarian cancer cases. An increase in the level of CA 72-4 can also be found in 6.7% of patients with a variety of benign diseases (pancreatitis, liver cirrhosis, pulmonary diseases, benign ovarian diseases, ovarian cysts, rheumatic diseases, gynecological diseases, benign diseases of the gastrointestinal tract).
CA 72-4 is used mainly in combination with CEA or CA-19-9 - to monitor the course and therapy of gastric cancer, or together with CA-125 - to monitor ovarian cancer, which increases the diagnostic sensitivity of testing.
Stomach cancer. There is no ideal tumor marker for gastric cancer yet. The literature provides data on the diagnostic sensitivity of the CA 72-4 test for this pathology from 28 to 80%, on average about 40 - 46%. The degree of increase in CA 72-4 levels correlates with the stage of the disease. After surgery, the CA 72-4 level returns to normal (on average within 3 to 4 weeks). This marker has a slightly higher sensitivity to subsequent relapses of the disease compared to CEA and CA 19–9. The complex use of these tests increases the diagnostic sensitivity and specificity of testing.
Ovarian cancer. Data on the diagnostic sensitivity of the test vary from 47 to 80%. The combined use of CA 72-4 and CA-125 increases the diagnostic sensitivity of the examination both during primary diagnosis and during further monitoring.
Colorectal cancer. The diagnostic sensitivity of the test is about 20–41%, while the level of the marker correlates with the clinical stage of the disease. After tumor resection, the concentration of CA 72-4 decreases markedly. During long-term follow-up, persistently elevated CA 72-4 concentrations are observed in cases of tumor remnants. The combined use of CA 72-4 and CEA increases the sensitivity of testing for disease relapse.
An increase in the concentration of Cyfra 21-1 is most typical for non-small cell lung cancer (NSCLC). But the lack of organ or tumor specificity of this marker does not allow us to recommend the Cyfra 21-1 study for screening lung cancer in the absence of clinical manifestations or in people at high risk of cancer. This study does not replace clinical and histological research methods in primary diagnosis, although it may be useful in cases where making a final diagnosis by biopsy is impossible for some reason.
Since most malignant lung tumors are heterogeneous in histological structure, the European Group on Tumor Markers (EGTM) recommends the use of 3 tumor markers for lung cancer, which are the most acceptable candidates for subsequent monitoring - neurospecific enolase (NSE, Cyfra 21-1 and carcinoembryonic antigen).
Tumor marker NSE
glycolytic enzyme (2-phospho-D-glycerate hydrolase, m.v. 80 kDa), existing in various variants of dimers, consisting of three immunologically distinct subunits (alpha, beta and gamma). The alpha subunit of enolase is detected in various tissues, the beta subunit is detected only in the heart and striated muscles. The alpha-gamma and gamma-gamma isoforms of enolase, referred to as neuron-specific enolase (NSE) or gamma enolase, were initially identified at high concentrations in neurons and neuroendocrine cells, as well as in tumors derived from these cells.
NSE is a specific serum marker of neuroendocrine tumors of APUD (Amine Precursor Uptake and Decarboxylation) cells, present in various tissues, and tumors associated with a neuroendocrine source. These tumors include neuroblastoma, retinoblastoma, medullary thyroid carcinoma, pancreatic islet cell carcinoma, carcinoid, pheochromocytoma, and small cell lung cancer. Enzyme activity correlates with clinical status and is used in disease monitoring and prognostic purposes (markedly elevated values indicate a poor prognosis), especially in small cell lung cancer and neuroblastoma.
Bronchial cancer. NSE is the main marker of small cell lung cancer (an increase is observed in 60–81% of all cases of small cell bronchial cancer). There is no correlation with the location of metastases or with cerebral metastases, but there is a good correlation with the clinical stage (progression) of this pathology.
In patients responding to chemotherapy, there is a transient increase in NSE levels 24 to 72 hours after the first therapeutic cycle, as a result of cytolysis of tumor cells. Within a week thereafter or at the end of the first cycle of therapy, there is a rapid drop in serum NSE concentrations (increased before the start of therapy) to reference values. In cases where there is no response to therapy, persistently elevated NSE levels are observed, or a decrease not to the level of reference values. During remission, 80–96% of patients show normal NSE values. An increase in marker concentration is observed in case of relapse (the latent period can be 1–4 months, the dynamics are exponential with a doubling period of 10–94 days, correlating with the prognosis).
In treatment monitoring, NSE can be used as the only prognostic factor (diagnostic sensitivity 93%, positive predictive value 92%).
Neuroblastoma . An increase in NSE is detected in 62% of children with this pathology. Height correlates with the stage of the disease.
APUDat home. An increased level of NSE is detected in 34% of cases (the marker can be used for monitoring purposes).
Seminoma. A clinically significant increase in NSE concentration is detected in 68–73% of patients, the degree of increase correlates with the stage of the disease.
Other tumors. Non-pulmonary malignant diseases are combined with an increase in NSE in 22% of cases, including brain tumors - only in some cases.
Benign diseases. An increase in NSE levels can also be observed in septic shock and pneumonia.
Nervous system. NSE is elevated in stroke, nervous system trauma, and benign brain diseases and is a poor prognosis for neurological deficits.
NSE is found in red blood cells, platelets and plasma cells, so serum should be separated from blood cells as quickly as possible (maximum within 1 hour).
Squamous cell carcinoma (SCC) antigen
Indications for the use of squamous cell carcinoma antigen (SCC) are monitoring the course and treatment of squamous cell carcinoma of the cervix, nasopharynx, ear, lungs and esophagus. Squamous cell carcinoma antigen (SCC) is a 42 kDa glycoprotein isolated from hepatic metastases of cervical squamous cell carcinoma.
The normal limit for squamous cell carcinoma antigen (SCC) is not higher than 2 ng/ml.
An increased level of squamous cell carcinoma antigen SCC is found in patients with squamous cell carcinoma of the cervix (up to 85%), with carcinoma of the nasopharynx and ear (up to 60%). An increase in the level of squamous cell carcinoma antigen (SCC) is detected in renal failure (up to 10 ng/ml), sometimes in patients with hepatobiliary pathology.
When studying this marker, you should pay attention to the collection of material and work with test samples, since contamination with elements of skin and saliva leads to false positive results.
Elevated levels of squamous cell carcinoma antigen (SCC) are found in both 31% of cases of squamous cell carcinoma of the lung and 17% of cases of non-small cell carcinoma.
Interpretation of results
Important! Standards vary depending on the reagents and equipment used in each particular laboratory.
Therefore, when interpreting the results, it is necessary to use the standards adopted in the laboratory where the analysis was carried out. You also need to pay attention to the units of measurement. To avoid errors when interpreting results over time, it is necessary to do the analysis in the same laboratory, since different institutions use different methods and reagents. In addition, conducting only one test for CA 72-4 and making an accurate diagnosis only on its basis is categorically unacceptable. It is required to conduct a comprehensive, comprehensive examination using additional diagnostic tests, including instrumental ones.
Reference values:
- 0-6.9 U/ml (Helix and Invitro laboratories);
- 0-4.6 IU/ml (handbook of laboratory diagnostics edited by MD, Prof. Kishkun)
Reliability and validity of tumor marker analysis results for gastric cancer
The reliability and validity of the results will depend on the following aspects:
- Features of collecting material and manipulating it before delivery to the laboratory.
- Diagnostic systems and equipment used for analysis.
- The presence of concomitant diseases that can lead to an increase in the concentration of the tumor markers being studied.
To obtain reliable results, it is recommended to take tests in accredited laboratories that have established pre-analytical and analytical stages of research, as well as internal and external quality control.
CA 72-4 increased
- Stomach cancer. Important! The concentration of CA 72-4 increases in proportion to the stage of the malignant process. Therefore, this analysis has a high prognostic value in assessing the risks of metastasis and assessing the patient’s chances of survival;
- Metastatic stomach cancer;
- Mucinous ovarian cancer;
- Oncology of the lungs, intestines, pancreas, mammary glands, endometrium;
- Liver pathology: hepatitis, cirrhosis;
- Benign ovarian neoplasms: cysts, polyps;
- Diseases of the gastrointestinal tract: gastric ulcer, pancreatitis (inflammation of the pancreas), etc.
Important! The interpretation of the results is always carried out comprehensively. It is impossible to make an accurate diagnosis based on only one analysis.
Causes of stomach cancer
How and why stomach cancer develops is not completely clear. But the influence of certain factors has been proven in which the likelihood of a tumor increases. These include:
- Chronic atrophic gastritis, especially against the background of hyperplastic processes of the epithelium. This disease was found in 60% of patients with stomach cancer. In this case, the localization of the process was important. For example, when atrophy is located in the antrum of the stomach, the risk of developing cancer increases 18 times, and with total damage to the organ - 90 times.
- Helicobacter Pylory infection. The likelihood of developing stomach cancer in such patients is 3-4 times higher than in the general population.
- Nutritional features. Containing a large amount of hot, spicy, pickled, salty foods, fast food, smoked meats and fats in the diet statistically significantly increases the likelihood of developing tumors of the gastrointestinal tract, including the stomach.
- Presence of adenomatous polyps. Such neoplasms have a relatively high risk of malignancy, so they are recommended to be removed in a timely manner.
- Smoking.
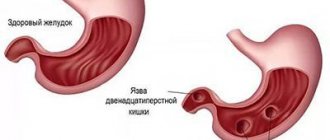
- History of gastric ulcer. When an ulcer is located in the body of the stomach, the risk of developing a malignant neoplasm increases by 2 times.
- History of gastric surgery. Increases the risk of developing cancer by 4 times.
- Menetrier's disease, which is characterized by hypertrophic gastropathy or hyperplastic giant-fold gastritis.
- Drunkenness and alcoholism.
- Pernicious anemia. Anemia with a malignant course, which develops due to the inability to absorb vitamin B 12. It is also accompanied by immunodeficiency conditions, which increases the risk of developing a malignant tumor by 10%.
- Hereditary predisposition. The presence of malignant stomach tumors in close blood relatives increases the risk of developing cancer in this location by 5-20%. It is believed that Napoleon Bonaparte and his father died from this disease.
- Immunodeficiency states.
- Working with carcinogenic substances.
Book a consultation 24 hours a day
+7+7+78