Acute pancreatitis (AP) is an initially aseptic inflammation of the pancreas, which can damage surrounding tissues, distant organs and systems.
Currently, the prevalence of acute pancreatitis is 34 cases per 100,000 population, the mortality rate from this disease is 1.16 per 100,000 population annually. In addition, in 20% of cases, repeated attacks of acute pancreatitis develop, which can lead to the development of diabetes and/or chronic pancreatitis [1].
Among the etiological reasons are the following [2]:
- impaired outflow of pancreatic secretions due to biliary hypertension (40%);
- long-term alcohol intake (30%);
- hyperglycerinemia (2–5%);
- other (autoimmune processes, vascular insufficiency, vasculitis, drugs (hypothiazide, steroid and non-steroidal hormones, mercaptopurine), infectious diseases (viral mumps, hepatitis, cytomegalovirus), allergic factors (varnishes, paints, odors of building materials, anaphylactic shock), dishormonal processes during pregnancy and menopause, diseases of nearby organs (gastroduodenitis, penetrating ulcer, tumors of the hepato-pancreatic-duodenal region).
The trigger mechanism for biliary AP is gallstones and gallbladder sludge syndrome in the area of the ampulla of Vater's papilla, which leads to an increase in pressure in the intrapancreatic ducts, accompanied by the accumulation of enzyme-rich fluid in the interstitial tissue of the gland. Since lipase is one of the few enzymes secreted in an active form, cell damage occurs [3].
Long-term intake of ethyl-containing products leads to hyperactivation of cholecystokinin, which causes premature activation of enzyme zymogens. In addition, alcohol enhances the damaging effect by activating the transcription factors nuclear factor kB ( NF-κB ) and activating protein-1(A-1). This leads to the formation of toxic metabolites - acetaldehyde and ethyl esters of fatty acids. Which, in turn, causes oxidative stress and disruption of the pancreatic parenchyma [4].
There are two theories to explain AP caused by hyperglycerinemia. According to the first, excess triglycerides are transported in the form of triglyceride-rich lipoproteins (chylomicrons), which are hydrolyzed in the vasculature of the pancreas. This releases a high amount of free fatty acids that albumin is unable to bind, leading to the formation of unbound fatty acids that damage platelets, endothelial cells and acinar cells. All this leads to acidosis, which increases the toxicity of free fatty acids and causes the activation of trypsinogen.
According to the second, an increased number of chylomicrons causes an increase in plasma viscosity, accompanied by impaired microcirculation, which causes ischemia and, as a consequence, acidosis and cell damage. It is likely that both of these theories complement each other (Fig. 1) [5].
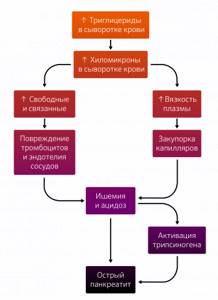
Figure 1 | Possible pathophysiological mechanisms of hyperglyceride pancreatitis
Naturally, there are many trigger mechanisms, as well as etiological reasons. However, you should not get hung up on them, since the pathogenesis of the leading causes is not fully understood, and rarely encountered forms are often presented as a bare theory. In addition, they often do not work alone: alcohol spasms the sphincter of Oddi, which causes intraductal hypertension; long-term alcohol intake leads to secondary hyperglycerinemia; and obesity is a risk factor for the development of gallstone disease. And, most importantly, regardless of the trigger, all pathways converge at one point—trypsinogen activation [4].
However, before continuing, we will make a slight digression to reveal the protective mechanisms of pancreatic cells, since intracellular activation of enzymes also occurs in a physiological state, and disruption of these mechanisms causes a significant part of idiopathic AP. Intracellular activation consists of [4]:
- pancreatic secretory trypsin inhibitor (PSTI or SPINK1), which binds and inactivates about 20% of the activated enzyme;
- autolysis of prematurely activated trypsin by autophagosomes;
- mesotrypsin and enzyme Y, which inactivate and lyse trypsin;
- nonspecific antiproteases such as alpha-1 antitrypsin and alpha-2-macroglobulins, which are present in the pancreatic interstitium.
Now let's return to pathogenesis. As a result of exposure to damaging factors, cell functioning is disrupted, which causes the initial stage of AP, which is divided into 3 components:
- intracellular activation of digestive enzymes;
- microcirculation disturbance;
- the appearance of inflammation.
Intracellular enzyme activation
As mentioned earlier, the activation of trypsinogen occupies a central position. At the moment, the main role is assigned to cathepsin B, a cysteine protease protein found in human lysosomes. Normally, the Golgi complex does not allow the fusion of vacuoles with digestive enzymes and lysosomes, but in a pathological condition, sorting is disrupted and their fusion occurs. Cathepsin B activates trypsinogen inside the vacuole. It is unable to withstand aggressive contents and breaks. A large amount of activated trypsin appears in the cell matrix, which the defense mechanisms are unable to cope with. Activated trypsin leads to the formation of more trypsin from trypsinogen, as well as to the “switching on” of other pancreatic enzymes, such as phospholipase, chymotrypsin and elastase, and dysregulation of other enzyme cascades, including complement, kallikrein-kinin and fibrinolysis.
Indications
Bile is synthesized by liver cells and collected through intrahepatic ducts in the gallbladder, which lies on the lower surface of the liver. Some time after eating, bile from the bladder is supplied to the duodenum, where it interacts with semi-digested food, breaking down its components into its component parts. The gallbladder and duodenum are connected by a common bile duct, into which the pancreatic duct flows just above the intestine.
During cholangiography, an X-ray contrast agent stains the extrahepatic ducts, showing their structure and patency, recording the sites of narrowing and the cause of the narrowing. Therefore, cholangiography is resorted to in the following cases:
- Jaundice of a non-infectious nature, when, due to a mechanical obstruction to the normal flow of bile, bile acids do not enter the intestine, but are absorbed into the blood, which is possible when the main bile duct - the common bile duct - is blocked by a stone, compressed by scars, as well as a tumor blocks the duodenal papilla - the confluence of the duct into the duodenum.
- Pancreatic cancer , when it is necessary to restore the patency of the common bile duct compressed by a cancerous conglomerate with a special drainage or a stent, so that the condition improves, obstructive jaundice regresses and the patient is prepared for a planned operation.
- Inflammation of the pancreas with frequent exacerbations to find the cause of the chronicity of the pathology.
- The presence of pathological purulent tracts - fistulas between the pancreas and duodenum, formed after severe pancreatitis.
After establishing the exact cause of bile excretion pathology, cholangiography from a diagnostic measure can become a therapeutic procedure that eliminates this pathological obstacle.

Inflammation
The immune system reacts to all this violence. Granulocytes and macrophages migrate to the site of damage. They begin to secrete pro-inflammatory substances:
- cytokines (tumor necrosis factor, interleukins 1, 6 and 8);
- arachidonic acid metabolites (prostaglandins, platelet-activating factor and leukotrienes);
- proteolytic and lipolytic enzymes;
- active oxygen metabolites that overload the already failing antioxidant system.
As a result, a significant number of damaging factors - activated enzymes, impaired blood supply, synthesis of pro-inflammatory mediators and excess oxides - leads to extensive death of pancreatic tissue and necrotizing pancreatitis occurs, developing in 20% of cases.
This ends the initial stage of the OP, which schematically looks like this (Fig. 2). Naturally, this is a model; in reality, pathological changes do not occur simultaneously; the condition of the organ is individual for each patient.
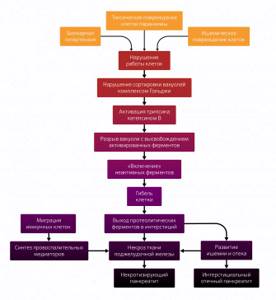
With a mild degree of AP, everything can end at the initial stage, but with the development of necrotizing pancreatitis, systemic changes in the body begin, which are clinically divided into two phases. Phase I is early, in turn divided into two periods.
Phase IA - the first week - the body is affected by aggressive proteolytic enzymes and a large number of inflammatory mediators that enter the systemic circulation, as well as hypovolemia, against the background of repeated vomiting, swelling and effusion into the serous cavities.
Overall, this leads to a metabolic shift in the form of hypocalcemia, hyperlipidemia, hyperglycemia/hypoglycemia and diabetic ketoacidosis, disseminated intravascular coagulation and a systemic inflammatory response.
By systems:
- lung damage is caused by the destruction of surfactant by phospholipase A against the background of DIC syndrome, which leads to respiratory distress syndrome;
- heart damage occurs as a result of a metabolic shift, hypovolemia and other factors that depress the myocardium, which leads to a drop in blood pressure up to shock;
- hypovolemia and a drop in blood pressure inhibit kidney function up to acute renal failure;
- in turn, the liver, trying to return the contents of the systemic bloodstream to the hemostatic ideal, cannot cope with the overwhelming work and breaks down into liver failure.
Separately here is the development of abdominal compartment syndrome. The impact of proteolytic enzymes, pro-inflammatory cytokines against the background of metabolic changes and disseminated intravascular coagulation syndrome on the cells of the serous membrane leads to active transudation of fluid into the internal spaces of the body. Developed ascites, as well as pronounced swelling of the parapancreatic tissue, lead to an increase in intra-abdominal pressure, which causes the development of abdominal compartment syndrome, which is an urgent surgical condition that causes the development of multiple organ failure [6].
This is how phase IA of necrotizing pancreatitis passes, which contains the first peak of AP mortality associated with organ or multiple organ failure.
Phase IB - usually the second week - is associated with the restoration of hemostasis and the body’s response to the formed foci of necrosis in the organ and periorgan tissue.
Then comes phase II - the late phase, the sequestration phase. Foci of necrosis undergo melting with the formation of pseudocysts and fistulas if the ductal system of the gland was located in the necrosis zone. This happens if sequestration is not accompanied by an infection.
The fact is that the first phase does not pass without leaving a trace on the gastrointestinal tract. Hypovolemia, a drop in blood pressure and the opening of arteriovenous shunts in the intestine, which occurs in response to a drop in blood pressure, lead to ischemia of the intestinal walls. This reduces its barrier function and makes it possible for bacteria to translocate from the lumen of the gastrointestinal tract to the mesenteric lymph nodes and more distant areas [7]. The contamination of tissues close to the pancreas causes such frequent infection - 20–40% [8]. It is the infectious process that causes the second peak of mortality associated with the development of the infectious process. After its relief, recovery begins.
AP most often begins acutely against the background of heavy food and/or alcohol intake or after an attack of acute hepatic colic - pain, nausea and repeated vomiting.
The pain is long-lasting (more than 30–60 minutes), severe, encircling in nature, located in the epigastrium without clear localization, aggravated when lying on the back and radiates into it in 50% of patients [9].
Physical manifestations depend on the severity of the disease. Upon examination, abdominal bloating, facial hyperemia, marbling or cyanosis of the skin of the abdomen and peripheral parts of the body are revealed. In very rare cases, with severe pancreatitis, Cullen's spots (Fig. 3), located paraumbilically, or Gray-Turner spots, located on the left side wall of the abdomen, may be observed. .
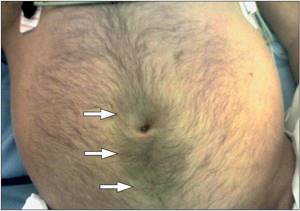
Palpation manifestations range from tenderness to severe muscle guarding and peritoneal syndromes. The Gubergrits symptom can help here - pain that occurs when pressure is applied to a point lying 6 cm above the navel, on the line connecting it to the left armpit. Janelidze's symptom - a decrease in pain with in-depth palpation of the epigastric region is characteristic of myocardial infarction, and an increase in pain - for acute pancreatitis. And the Mayo-Robson symptom is pain on palpation of the left costovertebral angle (projection of the tail of the pancreas). Percussion/auscultation reveals signs of intestinal paresis[10].
According to the Atlanta classification, to make a diagnosis of AP, the presence of two of the following three signs is required [11]:
- characteristic abdominal pain (acute, persistent, severe epigastric pain, often radiating to the back);
- Serum lipase (amylase) levels are at least 3 times the upper limit of normal;
- characteristic signs of AP on contrast-enhanced CT or, less commonly, magnetic resonance imaging (MRI) or transabdominal ultrasound.
A constant symptom is a characteristic pain syndrome. The situation is different with the other two criteria.
Lipase and amylase appear as a result of cell death. Moreover, amylase increases faster, reaching significant levels within a couple of hours. But it quickly returns to normal numbers, within 3–5 days, which makes it irrelevant if applied late. In addition, in 19% of cases of AP, the amylase level can remain normal in the initial period, and can also be high in conditions not associated with AP: macroamylasia, diseases of the salivary glands, in patients with reduced glomerular filtration, non-pancreatic inflammatory diseases of the abdominal organs : acute appendicitis, cholecystitis, acute intestinal obstruction, peptic ulcer, gynecological diseases [12]. The increase in lipase levels begins from 4 to 8 hours and reaches a maximum at 24 hours, and returns to normal within 8–14 days. It is also characterized by an increase in non-pancreatic pathologies: kidney disease, acute appendicitis, cholecystitis, intestinal obstruction, etc. [13].
In turn, CT may not detect changes at the initial stage, and gases in the distended intestine may mask focal hypoechoic zones within the pancreas [12]. But the combined use of laboratory and instrumental diagnostic methods ensures correct diagnosis in 81–95% of patients [14].
Once the diagnosis is made, the severity must be determined. It is divided into light, medium and heavy.
Mild is characterized by the absence of signs of local and general complications. It also corresponds to the morphological form of interstitial edematous pancreatitis.
The average degree is assigned to signs of organ failure, which resolves within 48 hours, in the presence or absence of local complications.
Signs of deficiency:
- respiratory system: Pao2 / FiO2 ≤ 300;
- cardiovascular system: systolic blood pressure <90 mm Hg. Art. (without inotropic support), despite fluid infusion, and/or pH < 7.3;
- kidneys: blood creatinine ≥ 170 mmol/l.
Local complications:
- acute peripancreatic fluid accumulation;
- pancreatic pseudocyst;
- quickly emerging zone of necrosis;
- impaired gastric emptying, thrombosis of the splenic and portal veins and necrosis of the colon.
Severe is diagnosed with persistent signs of organ failure (more than 48).
Moderate and severe degrees correspond to necrotizing pancreatitis, which in turn is divided into [15]:
- pancreatic parenchymal necrosis;
- peripancreatic necrosis;
- pancreatic parenchymal necrosis in combination with peripancreatic necrosis.
To determine the morphological form of AP and identify local complications, radiation diagnostics is necessary, which we will discuss in more detail.
Imaging for AP is usually necessary when the clinical situation is unclear. Using radiation research methods, it is possible to determine the main cause of AP, assess the complications and severity of the disease, and observe the dynamics of the development of the disease. But it is important to understand that visualization is not always recommended.
When to resort to radiation research methods [21]:
- Abdominal ultrasound - to identify and evaluate gallstones. The study should be performed on patients who have experienced such complaints for the first time and there is no identified pathology;
- in an acute situation, if the clinical picture and amylase and lipase levels are ambiguous, a contrast-enhanced CT scan should be performed;
- also, if the patient’s condition significantly deteriorates (sharp drop in hemoglobin and hematocrit levels, tachycardia, hypotension, sudden change in temperature or leukocytosis), CT with contrast is recommended.
Delayed CT with contrast injection (>7–21 days after symptom onset) is very effective in assessing severity and further management of the patient.
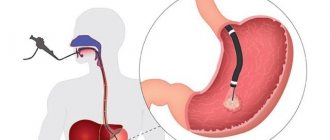
Endoscopic retrograde cholangiopancreatography (ERCP) - for biliary pancreatitis within the first 24 hours, and in patients with acute cholangitis - urgent ERCP (< 24 hours).
When to avoid radiation methods:
- with a clear (and obvious) clinical picture of acute pancreatitis;
- CT scan in the first 72 hours cannot reliably determine the severity of AP. Therefore, imaging should not be used to predict the severity of AP in the early stages of the disease;
- routine CT for initial assessment should not be performed, since the vast majority of complications can be suspected by clinical and biochemical signs;
- MR cholangiography and ERCP are generally not indicated for patients with mild biliary pancreatitis without clinical signs of obstruction of the common bile duct[21].
In the case of idiopathic AP, it is necessary to exclude biliary etiology using two ultrasound examinations of the abdominal cavity, and, if necessary, using RCCP and/or endosonography. All this is done in order to prevent relapse of pancreatitis.
Medical care for AP depends on the stage of its provision.
1. Pre-hospital stage. The main task is to transport the patient to the hospital. Until the moment of medical evacuation of the patient, the rule “hunger, cold and rest” is most relevant. It is worth saying that the first point, hunger, is not a therapeutic manipulation, but a necessary measure of the pre-hospital stage: fasting is needed to facilitate instrumental diagnosis in a hospital setting.
If you are an emergency worker, you must establish peripheral venous access and begin fluid resuscitation.
2. Hospital stage. The main task is to decide on management tactics and cure the patient.
It all starts with determining the severity. A patient with mild AP can be treated in the surgical department, while moderately severe patients are treated in the intensive care unit.
To assess the severity of AP and prognosis for the development of the disease in Europe, many different scales are used: Ranson, BISAP, APACHE-II and others. With Ranson scale values ≥ 3, BISAP ≥ 2, APACHE–II ≥ 8, severe AP can be diagnosed [16].
Table 1 |
Ranson AP severity scale


The BISAP scale can be used in cases where a patient with AP is already being monitored for this disease . BISAP will help monitor treatment and, if necessary, transfer the patient to the ICU.
Russian clinical guidelines of the Society of Surgeons (ROS) recommend using in practice a rating scale that was developed at the Research Institute of Emergency Medicine named after I.I. Dzhanelidze:
Table 3 | Scale for express assessment of the severity of AP at the Research Institute of Emergency Medicine named after. I.I. Dzhanelidze

Treatment of acute pancreatitis can be considered from a general and specific perspective. First, let's discuss general points.
Use of antibiotics
Recent studies have shown that antibiotic prophylaxis in patients with AP does not reduce mortality rates. Therefore, routine antibiotic prophylaxis is no longer indicated for all patients with acute pancreatitis [17].
Antibacterial therapy is recommended for the treatment of infected severe acute pancreatitis. However, diagnosis of infection may be difficult due to the clinical presentation, which may be similar to other infectious complications or to the inflammatory response caused by AP. In this case, measuring serum procalcitonin levels may help predict the risk of developing infected pancreatic necrosis.
Patients with infected necrosis should be treated with antibiotics whose spectrum of action includes both aerobic and anaerobic microorganisms.
Routine prophylactic use of antifungals is not recommended for patients with infected acute pancreatic necrosis, although Candida spp. occur in patients with infected pancreatic necrosis and indicate patients at higher risk of mortality.
As we said earlier, in the pathogenesis of AP, seeding of the tissues of the gastrointestinal tract occurs through the translocation of bacteria from the intestinal lumen, which causes infection of the digestive tract by microflora (E. coli, Bacteroides, Enterobacter, Klebsiella, S. faecalis, S. epidermidis and S aureus), which makes carbapenems highly effective, especially imipenem/cilastatin. In addition, quinolones, metronidazole and cephalosporins in high doses may be effective.
ERCP technique
The combination of endoscopy with x-ray examination is optimal in all respects: the highest possible diagnosis and accessibility for inspection of “secluded corners” while recording the entire process of identifying pathology on an x-ray and disk, which will allow subsequent consultation by involved specialists not blindly, but by video.
The first step is sequential endoscopy of the esophagus, stomach, and duodenum. Next, a catheter is inserted through a special channel of the endoscope, the tip of which is directed into the common bile duct, where the contrast agent is supplied. The location of the contrast in the anatomical area is filmed on X-ray film, and the process of movement of the endoscopic equipment is recorded on video. If any neoplasm is detected inside the intestine or duct, a piece of tissue is taken with tweezers - a biopsy; if a stone blocks the duct, it is also crumbled with tweezers and removed. If the duct is narrowed, a stent or drainage catheter is installed.
Infusion therapy and analgesia
Isotonic crystalloids are the preferred liquid. The volume should be adjusted based on the patient's age, weight, and pre-existing renal and/or cardiac disease. Hematocrit, BUN, creatinine, and lactate are laboratory markers of volume volume and adequate tissue perfusion and should be monitored. Ringer's lactate may be associated with an anti-inflammatory effect, but evidence for superiority of Ringer's lactate over isotonic saline based on randomized trials appears inconclusive.
Non-steroidal anti-inflammatory drugs (NSAIDs) are recommended as analgesics. However, they must be abandoned if the patient has acute kidney injury (AKI).
Epidural analgesia should be an alternative or agonist to intravenous analgesia. Patient-controlled analgesia (PCA) should be integrated into the patient's treatment strategy.
Dilaudid is preferred over morphine or fentanyl in the nonintubated patient.