© Author: A. Olesya Valerievna, candidate of medical sciences, practicing physician, teacher at a medical university, especially for SosudInfo.ru (about the authors)
It is impossible to imagine an area of medicine where additional examination methods would not be used. Ultrasound, due to its safety and informativeness, is especially actively used in many diseases. Doppler measurements are an opportunity not only to assess the size and structure of organs, but also to record the features of moving objects, in particular, blood flow.
Ultrasound examination in obstetrics provides a huge amount of information regarding the development of the fetus; with its help, it has become possible to determine not only the number of embryos, their gender and structural features, but also to observe the nature of blood circulation in the placenta, fetal vessels and heart.
There is an opinion that examining expectant mothers using the ultrasound method can harm the unborn baby, and with Doppler ultrasound the radiation intensity is even higher, so some pregnant women are afraid and even refuse the procedure. However, many years of experience in using ultrasound allows us to reliably judge that it is absolutely safe, and such a quantity of information about the condition of the fetus cannot be obtained by any other non-invasive method.
All pregnant women should undergo Doppler ultrasound in the third trimester; if indicated, it may be prescribed earlier. Based on this study, the doctor excludes or confirms a pathology, the early diagnosis of which makes it possible to begin treatment in a timely manner and prevent many dangerous complications for the growing fetus and mother.
What is Doppler for pregnant women on ultrasound?
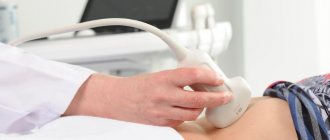
This diagnostic method is based on the Doppler effect, when the oscillation frequency of ultrasonic waves changes depending on the speed of movement of the objects under study. The examination is performed using an ultrasound machine with a special Doppler sensor. It records the reflection of ultrasound waves from blood cells that circulate through the blood vessels of the fetus, uterus and placenta. The device then processes the received data and converts it into a color image displayed on the monitor.
Why is Doppler prescribed during pregnancy? During the procedure, the doctor measures the direction and speed of blood flow, the location of blood vessels and their patency. This makes it possible to assess the state of the child’s circulatory system and shows possible disorders of the uteroplacental circulation.
What Doppler shows during pregnancy:
- The child’s heart rate and the work of his heart muscle;
- patency of blood vessels;
- condition of the heart valves;
- uteroplacental circulation;
- volume and speed of blood movement through the vessels;
- the functioning of the mother's cardiovascular system.
Many patients who are faced with such an examination for the first time are also concerned about the question of how Doppler is done during pregnancy. Diagnostics is carried out in the form of duplex or triplex scanning. The first option is used to analyze the blood flow of large vessels, their patency and identify the causes of deviations. The second Doppler method, also called color mapping, is considered more informative. It shows the condition of the smallest vessels, which makes it possible to recognize pathology at an early stage of development.
Severity of disturbance of uteroplacental blood flow
To determine the degree of disturbance of uteroplacental and fetal blood flow, there is a classification created under the editorship of prof. M.V. Medvedev, which allows one to judge the severity of blood flow disturbances. According to the classification, there are three degrees of violation of MPC:
- 1st degree (initial violations of MPC)
- 2nd degree (hemodynamic disturbances that do not reach critical values, but require drug correction)
- Grade 3 (critical blood flow disturbances, indicating intrauterine suffering of the fetus)
Regardless of the indicators of uteroplacental and fetal placental blood flow, it is also necessary to evaluate fetal blood flow. Assessment of blood flow velocity and vascular resistance in the middle cerebral artery reflects the presence or absence of oxygen starvation of the fetus (hypoxia), and will also help to exclude anemia in the fetus with the development of an immunohematological conflict according to the ABO system or the Rh factor. When assessing fetal blood flow, three degrees of disturbances are also distinguished:
- 1st degree – initial changes, i.e. reversible with timely treatment
- Grade 2 – signs of intrauterine hypoxia, requiring intensive care, possibly emergency delivery
- Grade 3 – signs of severe hypoxia, requiring an immediate decision on emergency delivery
Currently, when pregnant women are under the constant and close supervision of obstetricians and gynecologists, critical disorders of the uteroplacental and fetal blood flow, thanks to preventive measures and detection of changes in blood flow at the initial stages, are recorded very rarely. As a rule, severe changes in BMD are recorded in fetuses with genetic (chromosomal, gene) pathology, which is the basis for medical genetic counseling.
It should also be understood that women who are at high risk for various pregnancy pathologies must take responsibility for their health, lead a healthy lifestyle and visit their doctor in a timely manner. Only this gives every chance of having a healthy, full-term baby. You can find out the cost of Doppler ultrasound at the Vitromed clinic in the “Prices” section.
What is the difference between Doppler and ultrasound?
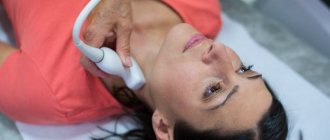
Doppler testing feels no different from an ultrasound examination, which shows the condition of the internal organs. The procedure is painless and takes about 20-40 minutes. To carry out the diagnosis, the pregnant woman lies down on the couch. The doctor applies hypoallergenic media gel to the patient's skin, then places the sensor on her abdomen. After measuring the main indicators, the specialist studies the results, compares them with standards and makes a transcript of the examination.
The main difference between a Doppler study and a conventional ultrasound is that the device generates ultrasound waves of a certain frequency, which are reflected only from moving objects - red blood cells and other blood cells that are in constant motion.
Indications
If the pregnancy proceeds without complications, the doctor may recommend that the patient undergo Doppler ultrasound during a routine ultrasound examination. If problems with the intrauterine development of the child are identified, diagnosis must be done immediately, regardless of the gestational age.
Doppler examination during pregnancy is prescribed in the following cases:
- discrepancy between the child’s weight and gestational age;
- suspicion of fetal hypoxia;
- fetoplacental insufficiency;
- anemia, diabetes mellitus and other chronic diseases in the mother;
- multiple pregnancy;
- polyhydramnios/oligohydramnios;
- immunological incompatibility of mother and child (Rh conflict);
- non-immune hydrops fetalis;
- early aging of the placenta;
- decreased motor activity of the child or complete absence of movements;
- fetal chromosomal abnormalities;
- history of pregnancy problems;
- gestosis.
Doppler ultrasound is also performed during pregnancy if the mother’s age is less than 17 or more than 35 years. As medical practice shows, the age factor increases the risk of developing fetal defects during pregnancy.
Timing of Doppler testing
It is recommended to do Doppler ultrasound no earlier than 18 weeks of pregnancy. By this time, the formation of the placenta is completed, and the doctor can objectively assess the state of the child’s circulatory system. Repeated diagnostics are done in the last trimester together with an ultrasound procedure. Why do you need a Doppler during pregnancy during this period? Diagnosis in the later stages shows abnormalities in the development of the fetus, entanglement of the umbilical cord or other abnormalities, which makes it possible to plan delivery with minimal risk to the health of the mother and baby.
If the pregnancy proceeds without disturbances, Doppler measurements are done twice - in the period 22-24 and 30-34 weeks. If there are medical indications, Doppler is prescribed unscheduled at any stage of gestation.
Preparing for Doppler testing
Many patients do not know how Doppler testing works during pregnancy and are worried about it. There is no need to worry, Doppler testing is an absolutely painless and safe procedure that does not require special preparation. When prescribing an examination, an obstetrician-gynecologist usually informs a woman about how Doppler is done for pregnant women, what it shows and how to prepare for the analysis.
The day before the scan, it is better for a pregnant woman to avoid eating foods high in fiber, which cause increased gas formation. Abdominal bloating during Doppler testing may interfere with an objective assessment of the state of the child’s circulatory system. Doctors also do not recommend drinking a lot of fluids and limiting food intake a couple of hours before the diagnosis so that the woman does not experience discomfort during the procedure.
If Doppler is done in a clinic, it is better for the pregnant woman to take a disposable diaper or towel with her to lay on the couch during the examination. As for private clinics, most of them provide patients with free napkins and disposable sheets, the cost of which is already included in the price list for Doppler testing.
What deviations occur?
In a healthy pregnancy that occurs without pathology, the movement of blood flow is not affected. If any complications are present, Doppler measurements of the vessels will reflect the picture of impaired hemodynamics in degrees:
- I degree, divided into indicators A and B: in the first case, the blood flow of the arteries approaching the uterus does not correspond to the norms, in the second - in the vessels going to the fetus;
- Grade II represents a disturbance in the blood flow of both the uterine arteries and the umbilical cord;
- Grade III is a threat to the life of the fetus and defines a critical failure in the blood supply.
What does Doppler show for pregnant women?
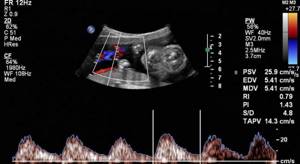
Doppler measurements show blood flow disturbances in a child in the early stages and allow us to determine the cause of their occurrence. Based on the diagnostic results, the doctor is able to prescribe timely treatment and adjust pregnancy management tactics.
During the procedure, the specialist evaluates:
- the degree of saturation of the child’s tissues with oxygen and nutrients;
- functionality of the vascular bed and possible deviations that impair blood flow;
- location and patency of the umbilical cord;
- condition of the placenta;
- the functioning of the patient's cardiovascular system.
Doppler results are generated by measuring the following indicators:
- average blood flow speed;
- pulsation index;
- systole-diastolic ratio;
- resistance index.
The doctor compares the obtained values with the standards and makes a transcript. If Doppler measurements correspond to the norm, it means that no pathologies of the uteroplacental circulation have been identified and the pregnancy proceeds without deviations. If the measurement results go beyond the acceptable limits, the specialist indicates in the conclusion all the abnormalities and possible causes of their occurrence, and also makes a prognosis for the further course of the pregnancy.
How are the results assessed?
Doppler testing used in gynecology evaluates the results according to the following parameters:
- resistance index, which is calculated as the difference between the maximum and minimum blood flow speed, divided by the maximum value of the same speed;
- systolic and diastolic ratio, that is, blood flow in different phases - systole and diastole;
- pulsation index, equal to the result of dividing the difference between the maximum and minimum by the average speed of blood flow.
The final indicators must correspond to accepted standards; each week of pregnancy has its own standard.
Standard Doppler indicators during pregnancy
Below, for general information, are presented the norms of the main indicators that are used when forming a medical opinion. It is important to know that deviations from standards do not always indicate the presence of pathologies. The final diagnosis is made by the doctor based on the gestational age, the possible range of fluctuations in indicators and other features of Doppler measurements.
Standard indicators of systole-diastolic ratio (SDR)
| Gestation period by week | SDO in the uterine arteries | SDO in the umbilical cord arteries | SDO in the middle cerebral artery of the fetus | SDO of the fetal aorta |
| 20-24 | up to 2.5 | up to 4.4 | from 2.9 | up to 8.4 |
| 25-27 | up to 2.4 | up to 3.8 | from 2.7 | up to 8.2 |
| 28-33 | up to 2.3 | up to 3.2 | from 2.4 | up to 7.9 |
| 34-41 | up to 2.3 | up to 2.9 | from 2.2 | up to 7.4 |
Average resistance index (RI) indicators
| Gestation period by week | IR of the uterine arteries | IR of the umbilical cord arteries | Fetal aortic IR |
| 20 | 0,52 | 0,74 | 0,79 |
| 21 | 0,51 | 0,73 | |
| 22 | 0,5 | 0,72 | |
| 23 | 0,5 | 0,71 | |
| 24 | 0,5 | 0,7 | |
| 25 | 0,49 | 0,69 | |
| 26 | 0,49 | 0,68 | |
| 27 | 0,48 | 0,67 | |
| 28 | 0,48 | 0,66 | |
| 29 | 0,47 | 0,65 | |
| 30 | 0,46 | 0,64 | |
| 31 | 0,46 | 0,63 | |
| 32 | 0,45 | 0,62 | |
| 33 | 0,45 | 0,61 | |
| 34 | 0,45 | 0,6 | |
| 35 | 0,45 | 0,59 | 0,78 |
| 36 | 0,44 | 0,58 | |
| 37 | 0,44 | 0,57 | |
| 38 | 0,44 | 0,56 | |
| 39 | 0,43 | 0,55 | |
| 40 | 0,43 | 0,54 | |
| 41 | 0,43 | 0,53 |
Average pulsation index (PI) indicators
| Gestation period by week | PI of the uterine arteries | PI of the umbilical cord arteries | PI in the fetal middle cerebral artery | PI of the aorta |
| 20 | 1,54 | 1,45 | 1,83 | 1,79 |
| 21 | 1,47 | 1,35 | 1,87 | 1,79 |
| 22 | 1,41 | 1,35 | 1,91 | 1,79 |
| 23 | 1,35 | 1,25 | 1,93 | 1,8 |
| 24 | 1,3 | 1,12 | 1,96 | 1,8 |
| 25 | 1,25 | 1,15 | 1,97 | 1,81 |
| 26 | 1,2 | 1,01 | 1,98 | 1,81 |
| 27 | 1,16 | 1,01 | 1,99 | 1,82 |
| 28 | 1,12 | 1,05 | 1,99 | 1,83 |
| 29 | 1,08 | 1,03 | 1,99 | 1,82 |
| 30 | 1,05 | 0,95 | 1,98 | 1,81 |
| 31 | 1,02 | 0,85 | 1,97 | 1,81 |
| 32 | 0,99 | 0,84 | 1,95 | 1,8 |
| 33 | 0,97 | 0,84 | 1,93 | 1,8 |
| 34 | 0,95 | 0,83 | 1,9 | 1,79 |
| 35 | 0,94 | 0,81 | 1,86 | 1,79 |
| 36 | 0,92 | 0,81 | 1,82 | 1,79 |
| 37 | 0,92 | 0,81 | 1,78 | 1,92 |
| 38 | 0,91 | 0,74 | 1,73 | 1,93 |
| 39 | 0,91 | 0,74 | 1,67 | 1,94 |
| 40 | 0,91 | 0,74 | 1,61 | 1,94 |
| 41 | 0,92 | 0,74 | 1,55 | 1,95 |
Doppler blood flow in the vessels of the uterus as a prognostic factor in the treatment of infertility.
Comparative characteristics of various methods for studying the condition of the endometrium.An objective assessment of the condition of the endometrium is one of the decisive factors for the successful implementation of the IVF procedure. To date, our knowledge regarding many biological mechanisms, including factors of uterine blood flow, endometrial secretion of specific proteins and other factors that regulate and determine successful implantation, is still limited. Adequately rapid enlargement and differentiation of the endometrium during the proliferative phase of the cycle should be accompanied by timely secretory changes during the luteal phase of the menstrual cycle. These changes are influenced by hormonal factors. There are a number of methods that allow, with varying degrees of objectivity, to assess the changes occurring in the reproductive system during the menstrual cycle [1].
These include measuring basal temperature, determining the level of estradiol ( E
) and progesterone in blood serum, histological examination of endometrial biopsy.
These methods have long been known and are widely used in practice. However, the first two are not informative enough, and the third is invasive, which limits its use, especially when preparing a patient for IVF.
In recent years, the method of ultrasound monitoring of follicle growth and endometrial thickness has been widely used, which in combination with hormonal monitoring is used in ovulation stimulation (superovulation).
One of the alternative methods for studying the condition of the endometrium is Doppler study of the nature of blood flow in the endometrial vessels [24, 34]. An objective assessment of changes (quantitative and qualitative) during the menstrual cycle was carried out on the basis of determining the physical properties of the endometrium using ultrasound scanning [22, 41].
In medicine, the Doppler effect is used mainly to measure the speed of blood flow, and the reflecting surface in this case is blood cells and primarily red blood cells. Blood flow velocity curves mean a graphical representation of changes in the average instantaneous or maximum velocity throughout the cardiac cycle.
The systole-diastolic ratio, resistance index, and pulsation index reflect the vascular resistance of the peripheral part of the vascular bed. Its increase is reflected mainly in the decrease in the diastolic component of the Doppler spectrum of the blood flow curve, which leads to an increase in the numerical values of the above indices.
High-frequency transducers (6.5-7.5 MHz) installed in sensors for transvaginal examination made it possible not only to increase the resolution and visualization of uterine structures, but also to combine research in
-mode simultaneously with Doppler measurements [21, 32, 46, 56, 62]. Color flow imaging, as well as quantitative and qualitative analysis, made it possible to assess the impedance of blood flow in the uterine arteries, which made it possible to characterize uterine perfusion [34, 35, 53, 56].
Recently, a number of works have appeared that not only provide Doppler characteristics of blood flow occurring during the menstrual cycle [15, 34], but also attempt to link them with the effectiveness of IVF [26, 27, 61].
Structure and function of endometrial blood vessels
Endometrial blood vessels form a vascular network that has a number of specific features. Unlike the vascular system of other organs and tissues, which maintain constant function and structure throughout life, endometrial vascularization changes dynamically over a relatively short period of time—the menstrual cycle [43].
During the menstrual cycle, the endometrial vasculature is subject to structural and functional changes that are closely related to changes occurring directly in the endometrium. In the first phase of the menstrual cycle, intensive growth of endometrial vessels and preparation for embryo implantation occurs. If implantation does not occur, the endometrial vasculature undergoes regression. The blood supply to the endometrium is carried out through the radial arteries, which are formed as a result of the division of the arcuate arteries in the thickness of the myometrium. After passing through the border between the myometrium and the endometrium, the radial arteries form smaller diameter basal arteries, which supply the basal layer of the endometrium, and spiral arterioles, which reach the surface of the endometrium [18, 39, 40].
The spiral arteriole has a spring-like appearance, which becomes more pronounced during the secretory phase of the menstrual cycle. In their structure, spiral arterioles differ from other arterioles in the relatively smaller amount of elastin in the inner wall of the arteriole [18]. Each spiral arteriole supplies a 4–9 mm area of blood,[7] with little or no overlap between areas. Just below the surface of the endometrium, the spiral arterioles branch and form the subepithelial capillary plexus.
The anatomy of the basal endometrial blood supply remains relatively unchanged throughout the menstrual cycle. The functional layer of the endometrium and the architecture of its blood supply constantly change in response to the effects of sex steroids depending on the phase of the menstrual cycle.
Considering the individual stages of angiogenesis of the functional layer of the endometrium, a number of authors [31] identify four main stages in the process of vascular growth:
1) rupture of the base of the membrane of an existing vessel; 2) movement of endothelial cells to the site of rupture; 3) rapid increase in the number of endothelial cells; 4) formation of endothelial cells into a new vessel.
In the interval between the end of menstruation and ovulation, two different phases of vascular growth are clearly visible in the process of endometrial vascularization:
- the first begins during menstruation and is nothing more than the restoration of the damaged vascular network [39], - the second phase is characterized by the growth of the vessels of the functional layer together with the rest of the endometrium under the influence of estrogens during the proliferative phase [17, 51].
While the morphological changes occurring in the endometrium and its vascularization are beyond doubt, the issue of factors influencing the process of angiogenesis of endometrial vessels remains a matter of debate. Estrogens play a predominant role in the angiogenesis of endometrial vessels [17, 51], but vascular endothelial growth factor [12, 51], fibroblast growth factor [19, 25], and TGF
-a [29], interleukin (
IL
)-1 [52] and
IL
-6 [58, 59], epidermal growth factor and
IL
-8 [14], angiotensin-II [6], etc.
In most placental mammals, a limited increase in endometrial microvascular permeability is one of the first signs of implantation. Thus, in an experiment during the implantation period, an increase in endometrial vascular permeability was detected in rats [46], guinea pigs [16], sheep [10] and pigs [30]. Despite the identity of the reaction of endometrial vessels to the implantation of an embryo in mammals, there remain many unexplored physiological mechanisms and reasons for this phenomenon. In women at the site of embryo implantation on the 9th day after ovulation, Hertig et al. (1956) revealed endometrial edema. Using electron microscopy, it was shown that the main changes in the microvascular architecture of the endometrium of rats during the increase in vascular permeability induced by blastocyst insertion [44] begin by the end of the 5th day of pregnancy. On the 6th day of pregnancy, the absence of capillaries surrounding the blastocyst was revealed, and as a result of implantation, an avascular zone ~ 420-210 mm in diameter appears [45]. Oddly enough, it is precisely at the moment of high metabolic activity that the embryo becomes isolated from the maternal energy sources to ensure its vital functions. There are a number of hypotheses that explain this seemingly paradoxical phenomenon. According to one of them [17,51], the presence of an avascular zone at the site of embryo implantation makes it possible to immunologically weaken maternal tissues and thereby expand the possibilities of trophoblast invasion into endometrial tissue.
This reaction may be part of a mechanism to avoid immunological rejection of the blastocyst by the mother. The avascular zone of the implantation site is surrounded by vessels of relatively larger diameter (capillaries closest to the site of embryo implantation have an average diameter of 18.5 ± 2.5 μm compared to capillaries located slightly further from the implantation site - 7.5 ± 0. 4 µm). In addition, the rate of blood flow through these dilated or relatively large capillaries in the implantation zone of the embryo is significantly reduced. There is a periodic decrease in blood flow velocity to zero values. Using electron microscopy, aggregation and adhesion of blood cells (usually leukocytes) and/or their rotation near the walls of the dilated vessel in the area of the embryo implantation site were detected, which rarely occurs in capillaries of a similar diameter, distant from the implantation site.
A detailed ultrastructural study of the microvascularization of the human endometrium [42] showed that in the early proliferative phase its basement membrane, surrounded by capillaries, is relatively discontinuous. During the proliferative phase, the endometrial basement membrane becomes more complex. Endothelial cells differentiate progressively towards the middle of the secretory phase of the cycle. The data presented indicate dynamic changes in the architecture of microvascularization of the human endometrium during the menstrual cycle. Under the influence of various factors, these specific changes determine the process of embryo implantation. If implantation does not occur, regression of endometrial vessels begins 8-9 days after ovulation. For the first time R. Goswamy et al. [28] and R. Goswamy and P. Steptoe [27] hypothesized that decreased uterine perfusion plays an important role in the development of infertility in women. This suggested that assessing uterine artery blood flow parameters during assisted reproductive technology cycles could provide additional information in order to optimize their implementation.
Doppler measurements of blood flow in the vessels of the uterus in assisted reproduction programs.
Doppler measurements of blood flow in the uterine arteries, performed using color Doppler mapping, have been proposed as one of the physiological parameters for indirectly assessing the ability of the endometrium to implant an embryo. It has been proposed to evaluate the Doppler characteristics of blood flow in the uterine arteries and their branches located in the outer third of the myometrium [20].
R. Goswamy and P. Steptoe [27] showed that the characteristics of uterine blood flow are largely determined by the content of estrogens in the blood serum and the duration of their influence. Thus, with an increase in the concentration of estrogen in the blood, uterine perfusion also increased, and with a decrease in their concentration, it decreased. In addition, the intensity of blood flow in the uterine arteries increased again in parallel with the increase in the concentration of progesterone and estrogens in the luteal phase of the cycle. Recent studies have revealed significant differences in the vascular resistance of the uterine arteries in cases of luteal phase insufficiency compared with that in women with a normal ratio of hormones in the luteal phase of the cycle.
In this case, studies of uterine blood flow were carried out in parallel with an analysis of the concentration of progesterone in the blood serum and an endometrial biopsy in the second phase of the cycle [34, 60], which made it possible to correlate the level of serum hormones, the studied blood flow characteristics and changes occurring in the endometrium. These data can be confirmed by a study conducted by R. Achiron et al. [5] in women with premature depletion of ovarian function: with the use of hormone replacement therapy, Doppler characteristics of uterine blood flow significantly improve. However, talking about a reliable correlation between the level of estrogen in blood serum taken from the cubital vein and the nature of blood flow in the vessels of the uterus seems controversial [2, 3]. Thus, S. Kupesic et al. [35] showed that in PCOS (polycystic ovary syndrome), despite normal E
in the blood serum, resistance to blood flow in the uterine artery increases, which negatively affects embryo implantation.
Studies conducted by P. Serafini et al. [47] also did not confirm the assumption of a connection between the concentration of E
and progesterone with the intensity of blood flow in the vessels of the uterus.
In all women examined with comparable concentrations of steroid hormones in the blood and comparable dosages of gonadotropins, when similar concentrations of E
in the blood serum were achieved, different characteristics of uterine blood flow were identified.
S. Bassil et al. [8] when studying the content of E
in blood serum, revealed a significant relationship between its concentration and the intensity of uterine perfusion in IVF cycles using GnRH a and gonadotropins.
Similar data were obtained by other authors [11]. The administration of hCG caused a decrease in uterine blood flow and, accordingly, an increase in the resistance index ( RI
) for 48 hours, then with an increase in the secretion of progesterone and
E
, significant improvements were noted in the characteristics of uterine blood flow. The resistance index, determined 2 days before the administration of human menopausal gonadotropin (hMG; in cycles using GnRH a, “long protocol”), was higher in those patients in whom pregnancy did not occur.
Studying the effect of GnRH a, C. Battaglia et al. [9] showed that changes in uterine blood flow (the suppressive effect appeared on the 25th day of administration) are associated exclusively with hypoestrogenemia. These data have been confirmed by other researchers [5].
When measuring blood flow velocity in the uterine, radial, spiral and ovarian arteries during the periovulatory period in spontaneous and antiestrogens-induced cycles with confirmed ovulation, the best Doppler characteristics of blood flow in natural cycles were revealed [33].
It is noted that an increase in vascular resistance to blood flow is usually expressed in an increase in the pulsation index ( PI
), which significantly reduces the likelihood of pregnancy during IVF cycles [36, 54, 57, 62, 63].
Data obtained from measuring PI
of spiral arteries confirm these results [33].
It has been shown that there is a positive correlation between the concentration of E
in the blood serum and the amount of blood flow in the endometrium [34], however, with an increase in the concentration of progesterone, the correlation between the concentration of
E
and the level of blood flow disappears [23].
Similar data were obtained by A.N. Strizhakov and A.I. Davydov [4] during Doppler measurements of pelvic vessels in patients with internal endometriosis. These data can be interpreted as a result of insufficient local estrogenic effects on the myometrium and endometrium. When studying level E
in blood serum obtained from the ulnar, tubal and uterine veins, significant differences in these values were revealed [2, 3]. J. Zaidi et al. [63] noted significant differences in the Doppler characteristics of blood flow in the uterine vessels with different ultrasound structures of the endometrium (more frequent absence of blood flow in the basal arteries of the endometrium with a hyper-echoic ultrasound structure), and when subendometrial blood flow was not detected, implantation did not occur.
In a prospective study of Doppler characteristics of blood flow measured at the site of the first division of the branch of the uterine artery, P. Serafini et al. [47] concluded that diastolic blood flow and the multilayer structure of the endometrium are the most valuable prognostic criteria for assessing the likelihood of pregnancy during cycles using assisted reproductive technologies. Based on these data, using regression analysis, an equation was obtained to calculate the ultrasound implantation index of the uterus [48]. Resistance index ( RI
) is of less significance for the prognosis of the onset and development of pregnancy compared to the characteristics of diastolic uterine blood flow [47].
It was found that the threshold values of diastolic blood flow were statistically insignificant for predicting pregnancy compared with the average PI
determined in both uterine arteries [47].
P. Levi-Setti et al. [37] argue that the most valuable prognostic criterion for assessing the likelihood of pregnancy in IVF cycles is the measurement of the PI
before administration of the permissive dose of hMG.
PI
values less than or equal to 3.0 may be considered a favorable prognostic factor in IVF cycles.
Other authors [63] not only confirm these results, but also complement them: PI
from 2.0 to 3.0, measured before the introduction of the permissive dose of hMG, is considered as an indicator that not only affects the pregnancy rate, but also significantly increases the frequency implantation
When measuring PI
before the introduction of a permissive dose of hCG (or on the day of the LH peak in natural cycles),
PI
not exceeding 3.3 are considered prognostically favorable [11, 13].
As C. Steer et al. show. [55], PI
has a high correlation with biochemical markers of implantation, including 24 kDa protein,
E
and histological data of the endometrium. In addition, unsatisfactory Doppler characteristics of uterine blood flow were detected not only in patients with an unsuccessful IVF attempt, but also in patients with early miscarriage after IVF [53].
Thus, the measurement of Doppler blood flow indicators such as PI
and
RI
of the uterine vessels can be used to predict the possibility of pregnancy in IVF cycles.
On the other hand, M. Locci et al. [38] with Doppler measurements of blood flow in the uterine arteries did not find any differences in its indicators obtained in fertile women and in patients in whom pregnancy occurred as a result of assisted reproductive technologies. The use of modern ultrasound devices with high resolution makes it possible to assess blood flow not only in the uterine arteries, but also in smaller uterine vessels, which significantly increases the objectivity of the data obtained [34].
Doppler testing of the blood flow of the uterine vessels not only allows one to obtain characteristics of the blood flow, but also in 96% of cases makes it possible to obtain criteria for the readiness of the endometrium for embryo implantation. In addition, if there is insufficient blood supply to the endometrium, it is possible to carry out its timely correction for the successful completion of cycles of assisted reproductive technologies [49, 50].
Analysis of the data presented allows us to conclude that Doppler measurements of the uterine vessels provide additional information about the state of the woman’s reproductive system, and it can be assumed that it is a fairly objective method for predicting the possibility of pregnancy in women undergoing IVF. Thus, Doppler assessment of vascular blood flow makes it possible to judge the functional characteristics of the endometrium, which significantly complements its widely used anatomical characteristics.
B. Kamenetsky
International Center for Reproductive Medicine, St. Petersburg
Literature
1. Ailamazyan E.K., Potin V.V., Rulev V.V.
Diagnosis of ovarian hormonal insufficiency.
Current issues in the physiology and pathology of the female reproductive system. St. Petersburg 1995; 16-19. 2. Savitsky G.A.
Uterine fibroids.
St. Petersburg: Put 1994; 214. 3. Savitsky G.A.
Regulation of the level of estradiol and progesterone in the local bloodstream of the uterus and fallopian tube.
Bulletin of the USSR Academy of Medical Sciences 1990; 5: 43-46. 4. Strizhakov A.N., Davydov A.I.
Clinical transvaginal echography.
M 1994; 174. 5. Achiron R., Levran D., Sivan E. et al.
Endometrial blood flow response to hormone replacement therapy in women with premature ovarian failure: a transvaginal Doppler study.
Fertil Steril 1995; 63:3:550-554. 6. Ahmady O., Gad M., Sheimy R. et al.
Comparative study between sonography, pathology and UGP in women with perimenopausal bleeding.
Anticancer Res 1996; 16:4B:2309-2313. 7. Balash JS, Vanrell JA
Corpus luteum insufficiency and fertility: a matter of controversy.
Human Reprod 1987; 2: 557-567. 8. Bassil S., Magritte JP, Roth J. et al.
Uterine vascularity during stimulation and its correlation with implantation in in-vitro fertilization.
Human Reprod 1995; 10:6:1497-1501. 9. Battaglia C., Artini PG, Bencini S. et al.
Doppler analysis of uterine blood flow changes in spontaneous and medically induced menopause.
Gynecol Endocrinol 1995; 9:2:143-148. 10. Birnholz J., Hrozencik D.
Technical improvement for ultrasonic study of the endometrium.
Int J Fertil 1988; 33:3:194-200. 11. Bustillo M., Krysa LW, Coulam CB
Uterine receptivity in an oocyte donation program.
Hum Reprod 10:442–445. 12. Chan FY, Chau MT, Pun TC et al.
Limitations of transvaginal sonography and color Doppler imaging in the differentiation of endometrial carcinoma from benign lesions.
J Ultrasound Med 1994; 13:8:623-628. 13. Cohen JJ, Debache C, Pigeau F et al.
Sequential use of clomiphene citrate, human menopausal gonadotropin and human chorionic gonadotropin in human in vitro fertilization.
II. Study of luteal adequacy following aspiration of the preovulatory follicles. Fertil Steril 1984; 42: 360. 14. Coulam CB, Stern JJ, Soenksen DM et al.
Comparison of pulsatility indices on the day of oocyte retrieval and embryo transfer.
Hum Reprod 1995; 10: 82-84. 15. Davies DW, Jenkins JM, Anthony FW et al.
Biochemical monitoring during hormone replacement therapy cycles for transfer of cryopreserved embryos in patients with functional ovaries.
Hum Reprod 1991; 6:934-938. 16. de-Ziegler D., Bouchard P.
Understanding endometrial physiology and menstrual disorders in the 1990. Curr Opin Obstet Gynecol 1993;
5:3:378-388. 17. Eyster KM, Stouffer RL
Adenylate cyclase in the corpus luteum of the rhesus monkey.
II. Sensitivity to nucleotides, gonadotropins, catecholamines and non-hormonal activators. Endocrinology 1985; 116: 1552-1558. 18. Ferenczy A., Gelfand MM
Proliferation kinetics of human endometrium during the normal menstrual cycle.
Am J Obstet Gynecol 1979; 133:859-867. 19. Ferenczy A.
Anatomy and histology of the uterine corpus.
Blaustein's Pathology of the Genital Tract. Ed. T. G. Kurman. 3 ed. Springer-Verlag 1987; 6: 257-291. 20. Fleischer AC, Cullinan JA, Jones HW et al.
Serial assessment of adnexal masses with transvaginal color Doppler sonography.
Ultrasound Med Biol 1995; 21:4:435-441. 21. Fleischer AC, Herbert CM, Hill GA et al.
Transvaginal sonography of the endometrium during induced cycles.
J Ultrasound Med 1991; 10: 93-95. 22. Fleischer AC, Pittaway DE, Beard LA et al.
Sonographic depiction of endometrial changes occurring with ovulation induction.
J Ultrasound Med 1984; 3: 341-346. 23. Forrest TS, Elyaderani MK, Kuilenburg MI et al.
Cyclic endometrial changes: US assessment with histologic correlation.
Radiology 1988; 167: 233-237. 24. Fraser IS, Peek MJ
Effects of exogenous hormones on endometrial capillaries.
Steroid Hormones and Uterine Bleeding. Ed. N. J. Alexander, C. d'Arcangues. Washington: AAAS Press 1992; 131-134. 25. Frydman R., Testart J., Giacomini P. et al.
Hormonal and histological study of the luteal phase in women following aspiration of the preovulatory follicle.
Fertil Steril 1992; 38: 312-313. 26. Gonen Y., Casper RF
Prediction of implantation by the sonographic appearance of the endometrium during controlled ovarian stimulation for in vitro fertilization (IVF).
J In Vitro Fertil Embryo Transf 1990; 7: 146-152. 27. Goswamy RK, Steptoe PC
Doppler ultrasound studies of the uterine artery in spontaneous ovarian cycles.
Hum Reprod 1988; 3: 721-726. 28. Goswamy RK, Williams G., Steptoe PC
Decreased uterine perfusion—a cause of infertility.
Hum Reprod 1988; 3: 955-959. 29. Hitschmann F., Adler L.
Der Bau der Uterus-schleimhaut des geschlestsreifen Weibes mit besonderer Berrucksichtigung der Menstruation.
Monatschr Geburtsch Gynakl 1908; 27: 1. 30. Kemmann E., Gemzell G., Beinert B.
Treatment of patients with hyperprolactinemia.
Am J Obstet Gynecol 1977; 129:4:145-149. 31. Kiesel I., Rabe T., Schleef J.
Pulsatil secretory pattern of gonadotropins and steroid during the follicular and luteal phase of the menstrual cycle in women.
Pulsatile GnRH 1925. Procced. 3rd Ferring Symp. Haarlem 1986; 43-50. 32. Kupesic S.
The first three weeks assessed by transvaginal color Doppler.
J Perinat Med 1996; 24:4:301-317. 33. Kupesic S., Kurjak A.
The assessment of uterine and ovarian perfusion in infertile patients.
Eur J Obstet Gynecol Reprod Biol 1997; 71:2:151-154. 34. Kupesic S., Kurjak A.
Uterine and ovarian perfusion during the periovulatory period assessed by transvaginal color Doppler.
Fertil Steril 1993; 60:3:439-443. 35. Kupesic S., Kurjak A., Vujisic S. et al.
Luteal phase defect: comparison between Doppler velocimetry, histological and hormonal markers.
Ultrasound Obstet Gynecol 1997; 9:2:105-112. 36. Lessey BA, Yeh I., Castelbaum AJ et al.
Endometrial progesterone receptors and markers of uterine receptivity in the window of implantation.
Fertil Steril 1996; 65:3:477-483. 37. Levi-Setti PE, Rognoni G, Bozzo M et al.
Color-Doppler velocimetry of uterine arteries in pregnant and nonpregnant patients during multiovulation induction for IVF.
J Ass Reprod Genet 1995; 12: 7: 413-417. 38. Locci M., Nazzaro G., De-Placido G. et al.
Angiogenesis: a new diagnostic aspect of obstetric and gynecologic echography.
J Perinat Med 1993; 21: 6: 453-473. 39. Rajaniemi HJ, Ronnberg L., Kauppila A., Ylostalo P., Jalkanen M., Saastameinen J., Selander J.
Luteinizing hormone receptors in human ovarian follicles and corpora lutea during the menstrual cycle and pregnancy.
J Clin Endocrinol Metab 1981; 54: 307-313. 40. Rabinowitz R., Laufer N., Lewin A. et al.
The value of ultrasonographic endometrial measurement in the prediction of pregnancy following in-vitro fertilization.
Fertil Steril 1986; 45: 824-828. 41. Ramsey EM
Vascular anatomy.
Biology of the Uterus. Ed. R. M. Wynn. 2nd ed. New York - London: Plenum Press 1982; 59-76. 42. Ritchie WG
Sonographic evaluation of normal and induced ovulation.
Radiology 1986; 161:1:1-10. 43. Rogers P., Milne B., Trounson A.
A model to show uterine receptivity and embryo viability following ovarian stimulation for in vitro fertilization.
J In Vitro Fertil Embryo Transf 1986; 3: 93-98. 44. Rogers PAW
Structure and function of endometrial blood vessels.
Hum Reprod Update 1996; 2:1:57-62. 45. Rogers PAW, Murphy CR, Gannon BJ
Changes in the spatial organization of the uterine vasculature during implantation in the rat.
J Reprod Fertil 1982; 65: 211-214. 46. Savard K, Marsh JM, Rice BF
Gonadotrophins and ovarian steroidogenesis.
Res Prog Horm Res 1965; 21: 285-356. 47. Serafini P., Batzofin J., Nelson J. et al.
Sonographic uterine predictors of pregnancy in women undergoing ovulation induction for assisted reproductive treatments.
Fertil Steril 1994; 62:815-822. 48. Serafini P., Nelson J., Batzofin J. et al.
Preovulatory sonographic uterine receptivity index (SURI): usefulness as an indicator of pregnancy in women undergoing assisted reproductive treatments.
J Ultrasound Med 1995; 14: 10: 751-755. 49. Sharma V., Riddle A. et al.
An analysis of factors influencing the establishment of a clinical pregnancy in an ultrasound-based ambulatory IVF program.
Fertil Steril 1988; 49:3:468-478. 50. Sher G., Dodge S., Maassarani G. et al.
Management of suboptimal sonographic endometrial patterns in patients undergoing in-vitro fertilization and embryo transfer.
Hum Reprod 1993; 8: 347-349. 51. Sheth S, Hamper UM, McCollum ME et al.
Endometrial blood flow analysis in postmenopausal women: can it help differentiate benign from malignant causes of endometrial thickening?
Radiology 1995; 195:3:661-665. 52. Shuiling GA
Secretion of LH and FSH: modulation by GNRH and estrogens basis and clinical aspect.
Pulsatil GNRH. 3rd Ferring Symp. 1986; 29-42. 53. Soules MR, Steiner RA, Clifton DK et al.
Abnormal patterns of pulsatile luteinizing hormone in women with luteal phase deficiency.
Obstet Gynecol 1984; 63: 626-629. 54. Spernol R., Hecher K., Schwarzgruber J. et al.
Doppler flow measurements of the uterine artery.
A prognostic factor for success in treatment by IVF? J Ultrasound Med 1993; 14:4:175-177. 55. Steer CV, Campbell S, Tan SL et al.
The use of transvaginal color flow imaging after in vitro fertilization to identify optimal conditions before embryo transfer.
Fertil Steril 1992; 57: 372-376. 56. Steer CV, Tan SL, Dillon D. et al.
Vaginal color Doppler assessment of uterine artery impedance correlates with immunohistochemical markers of endometrial receptivity required for the implantation of an embryo.
Fertil Steril 1995; 63: 101-108. 57. Steer CV, Campbell S, Pampiglione JS et al.
Transvaginal color flow imaging of the uterine arteries during the ovarian and menstrual cycles.
Hum Reprod 1990; 5: 391-395. 58. Stuart B., Drumm J., FitzGerald DE et al.
Fetal blood flow velosity waveforms in normal pregnancy.
Br J Obstet Gynec 1980; 87:9:780-785. 59. Tabibzadeh S.
Human endometrium: an active site of cytokine production and action.
Endocr Rev 1991; 12: 272-290. 60. Tabibzadeh S., Kong QF, Babaknia A. et al.
Progressive rise in the expression of interleukin-6 in human endometrium during menstrual cycle is initiated during the implantation window.
Hum Reprod 1995; 10:10:2793-2799. 61. Tan SL, Zaidi J, Campbell S et al.
Blood flow changes in the ovarian and uterine arteries during the normal menstrual cycle.
Am J Obstet Gynecol 1996; 175:3:1:625-631. 62. Tekay A., Jouppila P.
Intraobserver variation in transvaginal Doppler blood flow measurements in benign ovarian tumors.
Ultrasound Obstet Gynecol 1997; 9:2:120-124. 63. Zaidi J., Campbell S., Pittrof R. et al.
Endometrial thickness, morphology, vascular penetration and velocimetry in predicting implantation in an in vitro fertilization program. Ultrasound Obstet Gynecol 1995; 6:3:191-198.
Congresses, symposiums, seminars.
10th Congress of the European Society of Gynecological Endoscopy Lisbon, Portugal
, November 21-24, 2001
International Symposium on Polycystic Ovaries Melbourne, Australia
, November 24, 2001
17th World Congress on Fertility and Sterility Melbourne, Australia
, November 24-December 1, 2001
VIII Endometriosis Congress San Diego, USA
, February 24-27, 2002
12th World Congress on IVF and Molecular Reproduction Buenos Aires, Argentina
, March 16-19, 2002
British Fertility Society meeting Oxford, England
, April 10-2, 2002
7th Congress of the European Society for Contraception Genoa, Italy
, April 10-13, 2002
IV International Symposium on Preimplantation Genetics Cyprus, Limassol,
April 11-13, 2002
34th International Congress on Pathophysiology of Pregnancy Balaton, Hungary
, June 27-30, 2002
II General Conference “Infertility in the 3rd millennium” Prague, Czech Republic
, November 16-17, 2002
18th European Congress of Obstetrics and Gynecology Lubeck, Germany
, June 11-14, 2003
18th World Congress on Fertility and Sterility Montreal, Canada
, May 23-28, 2004